Targeted delivery of RNA-based therapeutics enables functional analysis of macrophage subpopulations
Targeted delivery of RNA-based therapeutics enables functional analysis of macrophage subpopulations
Rasmussen, R. K.; Gudbergsson, J. M.; Mathiesen, H.; Strauss, L. M.; Moller, I. H.; Thomsen, M. B.; Kristensen, M. W.; Andersen, M. N.; Etzerodt, A.
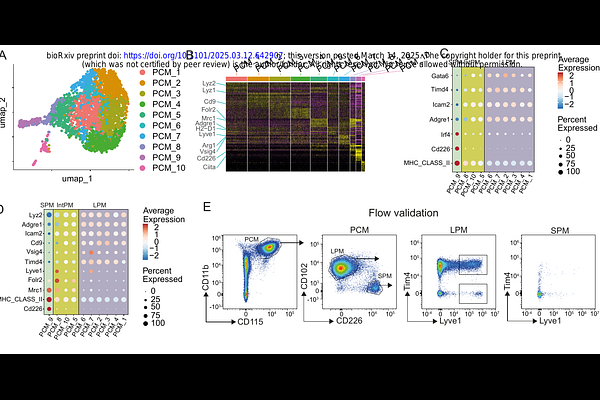
AbstractMacrophages infiltrate all human tissues where they play key roles in innate immunity, homeostasis, and tissue function. However, extensive clinical and experimental evidence indicates that macrophages also contribute significantly to the progression of several diseases such as cancer, cardiometabolic disorders, and inflammatory and neurodegenerative conditions. Advances in single-cell omics have revealed diverse macrophage populations in both healthy and diseased tissues. However, studying their functions is challenging due to limitations in tools for targeting specific populations. The Cre-lox system, involving Cre recombinase expression driven by macrophage-specific promoters, is widely used for gene manipulation. Despite its utility, this method has drawbacks like leaky expression, variable efficiency, and potential toxicity. Moreover, genetic models are costly and can have unintended effects on immune cells, hindering comprehensive studies on macrophage function. To address this challenge, we developed an advanced lipid nanoparticle-based system for precise RNA therapeutic delivery to macrophages, either broadly or via antibody-mediated targeting of specific subsets. This versatile platform enables the administration of various RNA molecules, such as mRNA, siRNA, and sgRNA for CRISPR/Cas9 applications, in both in vitro and in vivo settings. It allows for targeted cell depletion or gene knockout, facilitating detailed functional analysis. Furthermore, the system\'s flexibility and precision are enhanced by its compatibility with Cre-specific Cas9 expression, enabling comprehensive genomic and proteomic targeting of specific macrophage subsets.