Interleukin-22 in enteroendocrine cells controls early-life gut motility through interactions with the microbiota
Interleukin-22 in enteroendocrine cells controls early-life gut motility through interactions with the microbiota
Rabahi, S.; Marachlian, E.; Guendel, F.; Mikdache, A.; Quintero-Castillo, K.; Maurin, L.; Di Donato, V.; Janardhana Kurup, A.; Salloum, Y.; Diabangouaya, P.; Gros, G.; Garcia Baudino, C.; Medina-Yanez, I.; Levraud, J.-P.; Lutfalla, G.; Del Bene, F.; Boekhorst, J.; Feijoo, C.; Eberl, G.; Brugman, S.; Sumbre, G.; Hernandez, P. P.
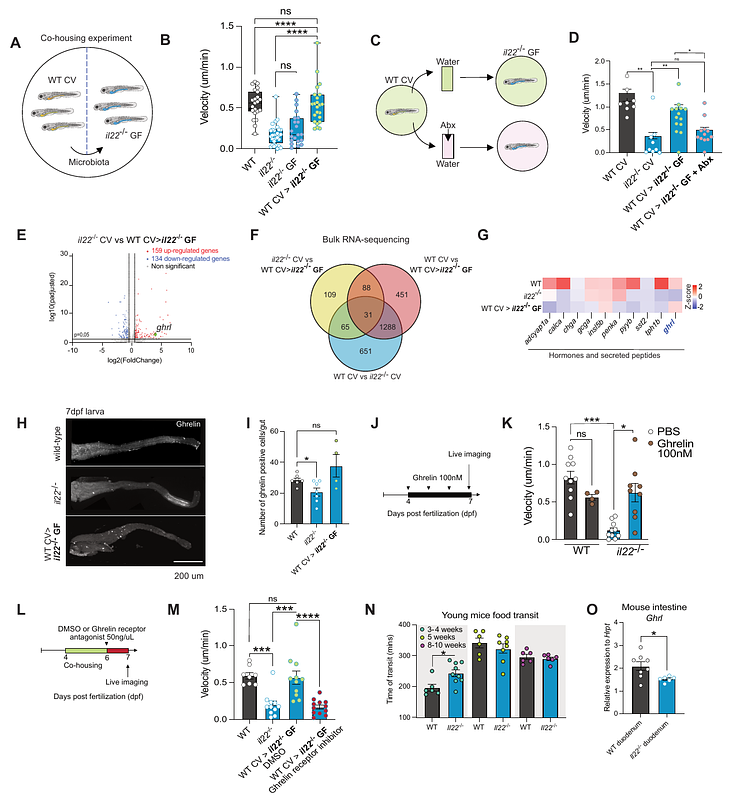
AbstractThe gut microbiota, immune system, and enteric nervous system tightly interact to regulate adult gut physiology. Yet the mechanisms establishing gut physiology during development remain unknown. Here, we report that in developing zebrafish, enteroendocrine cells produce IL-22 in response to microbial signals before lymphocytes populate the gut. In larvae, IL-22 is crucial to set gut microbiota composition, and ghrelin hormone expression to promote gut motility. IL-22 developmental function depends on its ability to modulate gut microbiota, as bacterial transfer from wild-type zebrafish restored gut motility in il22-/- by reestablishing ghrelin hormone expression. Additionally, IL-22-deficient mice show impaired gut motility and reduced ghrelin expression in early life, indicating a conserved function. Altogether, we identify a circuit where enteroendocrine cells regulate gut function via cytokine control of the microbiota, showing how gut physiology is set prior to immune system maturation.