T cell development from expanded hematopoietic progenitors reveals progression control by Lmo2, Erg, Spi1, Hoxa9, and Meis1
T cell development from expanded hematopoietic progenitors reveals progression control by Lmo2, Erg, Spi1, Hoxa9, and Meis1
Shin, B.; Chang, S. J.; MacNabb, B. W.; Sidwell, T.; Williams, B. A.; Rothenberg, E. V.
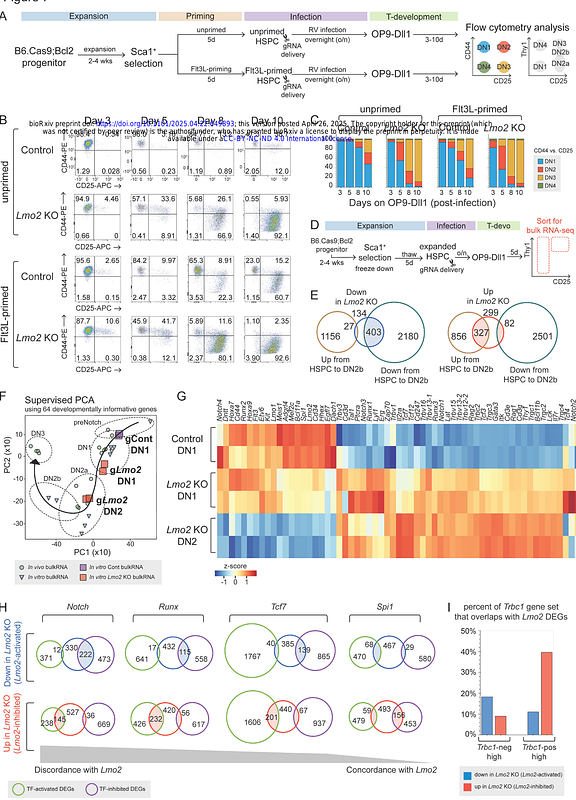
AbstractTo gain access to the earliest stages of T cell development, we adapted a serum-free culture system that expands hematopoietic stem and progenitor-like cells. These expanded cells efficiently undergo normal T-cell differentiation in vivo and in vitro, verified by early gene expression trajectories from single-cell RNA sequencing, though their absolute differentiation speed is slower than that of fresh progenitors and can be modulated with cytokine priming. Leveraging this expansion system to observe the first T-lineage events, we revealed that initial Notch activation immediately induces chromatin opening and transcriptional activation of the TCR-C{beta} locus. Additionally, acute CRISPR knockouts confirmed T-lineage entry requirements for Ikzf1, Hes1, Gabpa, and Myb while revealing that Lmo2, Erg, Spi1, Hoxa9, and Meis1 retard developmental progression with differing effects on proliferation. Endogenous expression of the stem, progenitor, and leukemia-associated factor Lmo2 markedly restrains initiation of the T cell program, with Lmo2 knockout greatly accelerating germline TCR{beta} locus transcription and expression of Tcf7, Gata3, Runx family, and E protein genes and their targets.