Defining the ordered pathway for ZAP-mediated RNA decay
Defining the ordered pathway for ZAP-mediated RNA decay
Bouton, C. R.; Gimpelj Domjanic, G.; Lista, M. J.; Galao, R. P.; Courty, T.; Wilson, H. D.; Hill, P. W. S.; Mischo, H. E.; Chakrabarti, A. M.; Poljak, M.; Ule, J.; Neil, S. J. D.; Swanson, C. M.
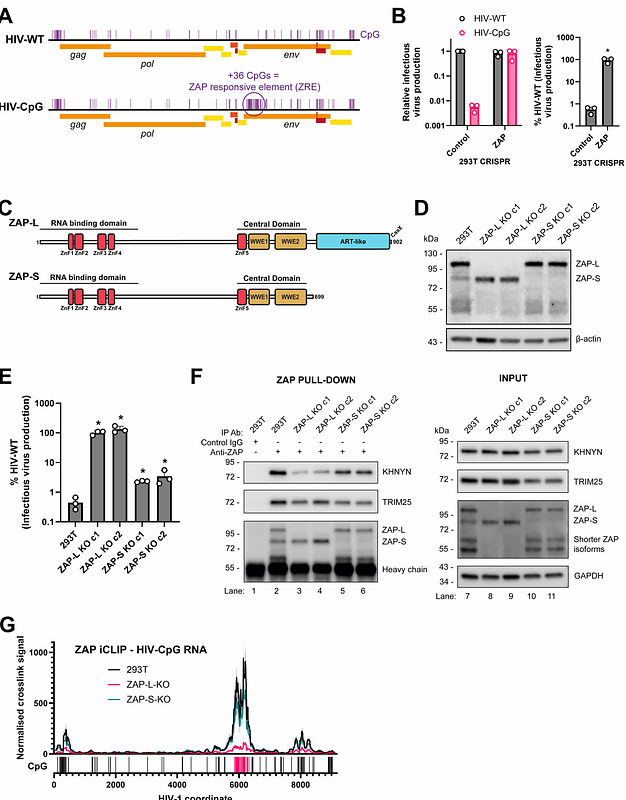
AbstractZinc-finger Antiviral Protein (ZAP)-mediated RNA decay (ZMD) restricts replication of viruses containing CpG dinucleotide clusters. However, why ZAP isoforms differ in antiviral activity and how they recruit cofactors to mediate RNA decay is unclear. Therefore, we determined the ordered events of the ZMD pathway. The long ZAP isoform preferentially binds viral RNA, which is promoted by TRIM25. The endoribonuclease KHNYN then cleaves viral RNA at positions of ZAP binding. The 5' cleavage fragment undergoes TUT4/TUT7-mediated 3' uridylation and degradation by DIS3L2. The 3' cleavage fragment is degraded by XRN1. ZAP and TRIM25 interact with KHNYN, TUT7, DIS3L2 and XRN1 in a RNase-resistant manner. Viral infection promotes the interaction between ZAP and TRIM25 with these enzymes, leading to viral RNA degradation while also decreasing the abundance of many cellular transcripts. Overall, the long isoform of ZAP recruits key enzymes to assemble an RNA decay complex on viral RNA.