Impact of dupilumab and upadacitinib treatment on epigenetic and transcriptomic alterations in skin-homing T cells of atopic dermatitis patients
Impact of dupilumab and upadacitinib treatment on epigenetic and transcriptomic alterations in skin-homing T cells of atopic dermatitis patients
Elfiky, A. M. I.; Smits, H. M.; van der Wal, M.; Dekkers, C.; Boesjes, C. M.; Drylewicz, J.; de Bruin-Weller, M.; van Wijk, F.
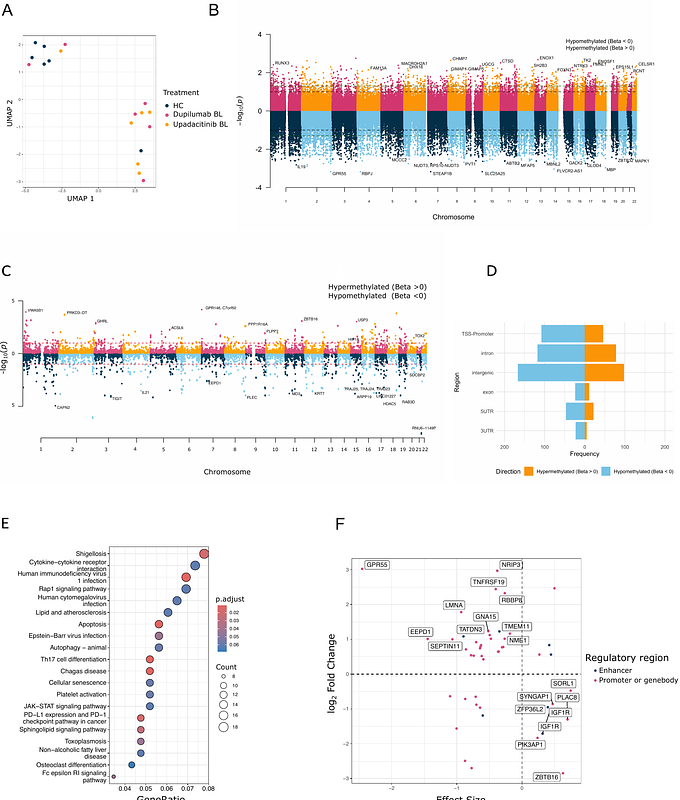
AbstractBackground: Biologic therapies, such as the IL-4 receptor alpha blocker dupilumab, and Janus kinase (JAK) inhibitors like upadacitinib, have revolutionized the management of atopic dermatitis (AD). However, prolonged treatment is necessary to sustain disease remission. While both therapies are clinically effective, only dupilumab has demonstrated successful dose reduction, suggesting potential disease-modifying effects. This study investigates the immune-modulating potential of one year treatment with dupilumab or upadacitinib in AD by assessing epigenetic and transcriptomic changes in skin-homing T cells. Methods: Using flow cytometry, CD4+CLA+ T cells were isolated from AD patients (n=12) at baseline and after 52 weeks of dupilumab (n=6) or upadacitinib (n=6) treatment. DNA methylome, RNA transcriptome and cytokines expression analyses were conducted using the EPIC array, RNA sequencing and flow cytometry, respectively. Non-atopic healthy controls (HC) (n=6) were included for comparison. Results: We identified 747 differentially methylated regions (DMRs) between HC and AD patients (p-value < 0.1), with a predominance of hypomethylation in AD. These DMR-associated genes were enriched in pathways such as cytokine-cytokine receptor interactions and JAK-STAT signaling. Several DMRs were associated with corresponding changes in the transcriptome and significant differential methylation was observed near 11 AD-associated SNPs. Following treatment, dupilumab partially corrected 6 AD-related DMRs towards the HC profile. Conversely, upadacitinib modulated 40 AD-related DMRs, most shifting further towards the AD profile. Intriguingly, 241 DMRs (upadacitinib) and 13 DMRs (dupilumab) were altered independently of AD-related DMRs, suggesting a broader epigenetic impact of upadacitinib. Conclusion: Our findings demonstrate distinct epigenetic effects of dupilumab and upadacitinib in skin-homing T cells from AD patients. The direction and extent of these modifications may contribute to differences in long-term disease control and have clinical implications for therapy discontinuation strategies.