Rational design and comparative docking and simulation of modified FLT3 inhibitors: A study on enhanced binding stability and inhibition potency
Rational design and comparative docking and simulation of modified FLT3 inhibitors: A study on enhanced binding stability and inhibition potency
Das, U.; Regati, D. R.; Sowdhamini, R.
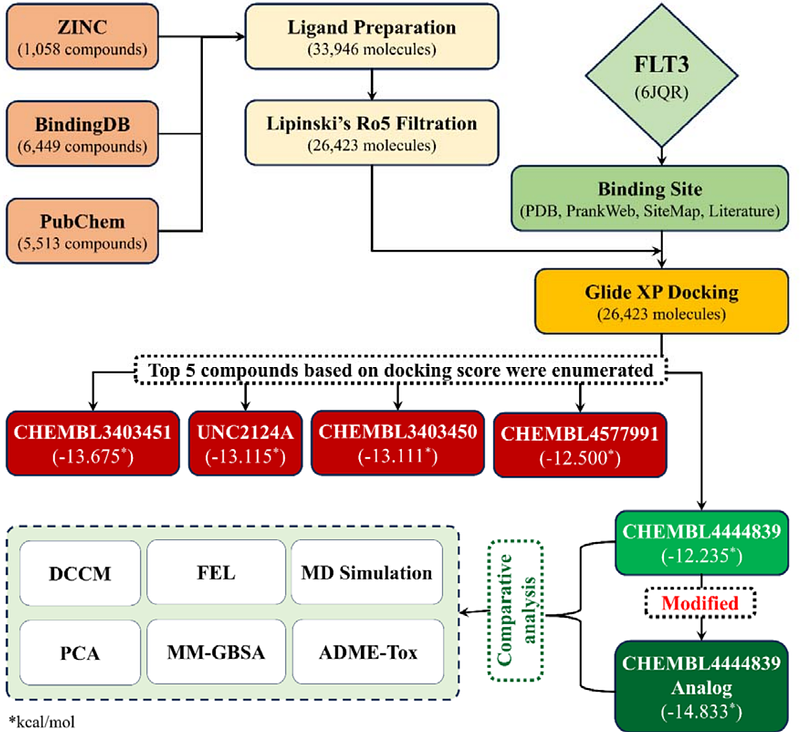
AbstractThe FLT3 protein is a well-established therapeutic target in the treatment of Acute Myeloid Leukemia (AML), with its inhibition playing a crucial role in disease management. In this study, we identify and propose a novel FLT3 inhibitor that demonstrates superior binding affinity, stability, and pharmacokinetic properties compared to currently available inhibitors. We initially characterized the binding interactions of known FLT3 inhibitors through molecular docking and then strategically modified functional groups to enhance binding affinity, optimize drug-likeness, and minimize toxicity. The resulting analogue exhibits improved metabolic stability, lower toxicity, higher intestinal absorption, and superior permeability. Molecular dynamics simulations further confirm that the novel inhibitor forms stable and persistent interactions with FLT3, as evidenced by reduced conformational fluctuations and compact structural integrity. Free energy calculations reveal stronger ligand stabilization, while dynamic correlation analysis suggests enhanced engagement with critical residues, reinforcing its potential as an effective therapeutic agent. These findings highlight a promising candidate for further experimental validation and potential development in AML treatment.