NOTCH1 Acts as a Tumor Suppressor That Induces Early Differentiation in Head and Neck Cancer
NOTCH1 Acts as a Tumor Suppressor That Induces Early Differentiation in Head and Neck Cancer
Huang, C.; Moorthy, S.; Li, Q.; Ahmed, K. M.; Deng, D.; Wang, J.; Rao, X.; Zhang, J.; Xi, Y.; Wang, J.; Liu, Z.; Tanaka, N.; Wheeler, D. A.; Shibrot, E.; Saade, R.; Pickering, C. R.; Xie, T.-X.; El-Naggar, A. K.; Osman, A. A.; Rai, K.; Zweidler-McKay, P. A.; Heymach, J. V.; Byers, L. A.; Johnson, F. M.; Sandulache, V. C.; Myers, J. N.; Yadollahi, P.; Frederick, M. J.
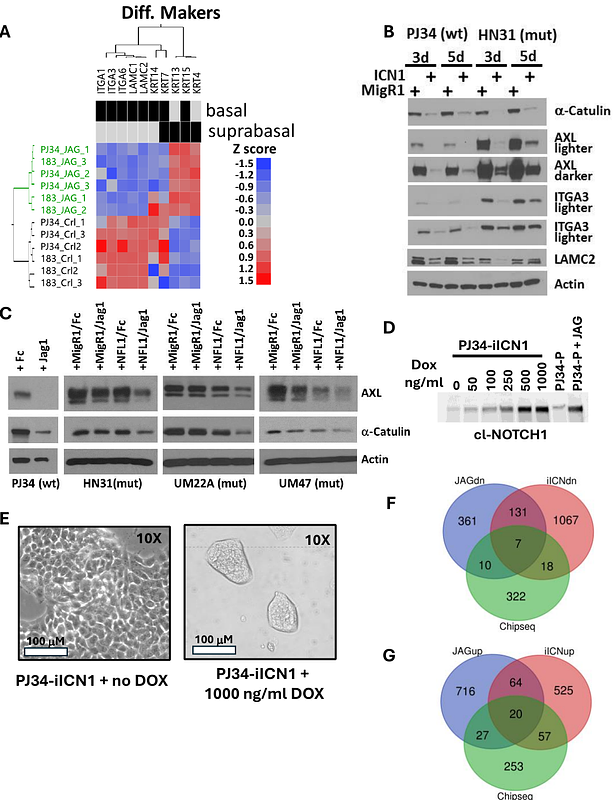
AbstractWe identified frequent inactivating notch1 mutations in HNSCC over a decade ago, indicating its role as a tumor suppressor, unlike its oncogenic function in leukemias and salivary gland tumors. However, there has been much debate in the literature regarding a possible oncogenic role in HNSCC as well, based on reports that notch1 signaling drives tumor growth and a cancer stem cell phenotype in some HNSCC tumor lines and patient samples. Clarifying whether NOTCH1 occasionally functions as an oncogenic driver in HNSCC is crucial to the prognosis and personalized therapy of patients with either wild-type or mutated NOTCH1. Here we present a systematic and comprehensive investigation unequivocally demonstrating that notch1 signaling functions as a tumor suppressor in HNSCC regardless of mutation or activation status and leads to reduction in frequency of cancer stem cells. We develop a robust gene expression signature of notch1 activation based on experimental data that when applied to patient samples shows notch1 signaling is associated with very early differentiation, an altered tumor microenvironment, and better prognosis, consistent with a tumor suppressive role. Our work unifies the field by reconciling conflicting data and providing critical insights into the biological and clinical significance of the NOTCH1 pathway in HNSCC.