Harnessing systemic glycolysis-TCA cycle axis to boost the host defense against newborn infection
Harnessing systemic glycolysis-TCA cycle axis to boost the host defense against newborn infection
Wu, Z.; Tien, N. T. N.; Klabunde, B.; Aasmul-Olsen, K.; Offersen, S. M.; Yen, N. T. H.; Muk, T.; Thysen, A. H.; Brix, S.; Brustad, N.; Wang, T.; Stokholm, J.; Bonnelykke, K.; Brunse, A.; Long, N. P.; Chawes, B.; Baek, O.; Nguyen, D. N.
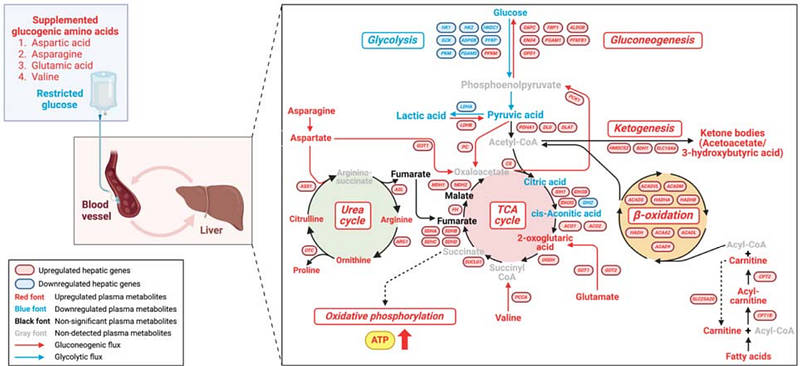
AbstractEnergy metabolism and immune response are tightly connected, but it is poorly understood how this interplay is regulated in early life to dictate host defense strategy, infection risks and severity. This interplay is particularly relevant for preterm, low birthweight or otherwise immunocompromised infants, who have poor metabolic control and increased risks of sepsis. Here, we utilized data from the COPSAC2010 cohort with 700 mother-child pairs and showed that plasma levels of TCA cycle metabolites in early life were associated with reduced childhood risk of bacterial infection and an attenuated systemic inflammatory response. Next, we explored how two distinct nutritional strategies, which were aimed at boosting TCA cycle activity instead of glycolysis, impacted neonatal host defense against a serious bloodstream infection in preterm piglets. Substituting galactose for glucose in parenteral nutrition enhanced disease tolerance in early phase of infection and overall glucose homeostasis, improving survival. Further, combining glucose restriction with supplementation of glucogenic amino acids conferred glycemic control and completely prevented sepsis and abnormal changes of organ injury markers. Mechanistically, this intervention enhanced both disease resistance and tolerance, accompanied by metabolic rewiring from glycolysis towards gluconeogenesis, TCA cycle activity and oxidative phosphorylation. Thus, optimized nutritional strategies controlling the interplay of energy metabolism and host defense may be lifesaving for infected infants.\n\nIn briefNewborns rely on two distinct defense strategies to combat infections in early life. Disease resistance, fueled by aerobic glycolysis, seeks to actively eliminate microorganisms while disease tolerance, fueled by mitochondrial oxidative phosphorylation, seeks to reduce collateral tissue damage during infections. We found that in healthy human newborns, increased plasma levels of metabolites from the tricarboxylic acid (TCA) cycle were associated with lower burden of childhood infections and reduced pro-inflammatory status. In a newborn animal model of bloodstream infection, nutritional strategies boosting systemic TCA cycle activity, while reducing aerobic glycolysis, enhanced both host disease tolerance and resistance, thereby improving survival. These findings could pave a path for improved infection management in human newborns.\n\nHighlightsO_LIIn healthy children, higher plasma levels of TCA cycle metabolites are associated with lower infection risks and systemic inflammation.\nC_LIO_LIIn a neonatal infection model, the supply of galactose, instead of glucose, improves host glucose homeostasis and TCA cycle activity, improving disease tolerance and survival.\nC_LIO_LIA combination of glucose restriction and glucogenic amino acid supply also improves TCA cycle activity, enhancing both disease resistance and tolerance and completely preventing lethal sepsis.\nC_LI