An Enantioselective Chemical Probe for Chikungunya nsP2 Helicase with Antialphaviral Activity
An Enantioselective Chemical Probe for Chikungunya nsP2 Helicase with Antialphaviral Activity
Ramalingam, M. B.; Oh, H. J.; Sears, J. D.; Chen, C.-H.; Vala, A.; Liu, S.; Talbot, K. M.; Hossain, M. A.; Brown, P. J.; Houliston, S.; Garcia Perez, J.; Li, F.; Amare, M. G.; Halfmann, P.; Smith, J.; Hirsch, A.; Arrowsmith, C. H.; Halabelian, L.; Vargason, A. M.; Counago, R. M.; Arnold, J. J.; Cameron, C. E.; Moorman, N. J.; Heise, M. T.; Willson, T. M.
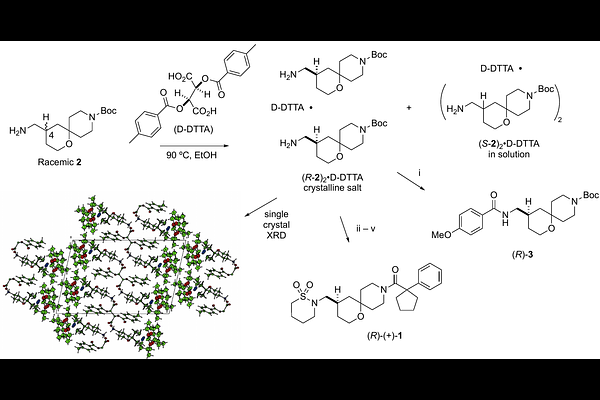
AbstractChikungunya virus (CHIKV) replication relies on the multifunctional nsP2 protein, making it an attractive target for antiviral drug discovery. Here, we report the resolution of oxaspiropiperidine 1, a first-in-class inhibitor of the CHIKV nsP2 RNA helicase (nsP2hel), into its constitutive enantiomers and characterization of their antiviral activity. The enantiomer (R)-1 exhibited potent inhibition of viral replication, nsP2hel ATPase activity, and dsRNA unwinding, while the (S)-1 enantiomer was >100-fold less active. The (R)-1 enantiomer also demonstrated high selectivity for CHIKV over other RNA viruses and for nsP2hel over other RNA helicases. Direct binding of (R)-1 to nsP2hel protein was confirmed by 19F NMR. Biophysical and structural studies revealed conformational polymorphism in the spirocyclic scaffold of (R)-1, suggesting a potential role of thermal mobility of the ligand in allosteric inhibition of nsP2hel. Collectively, these findings designate (R)-1 (RA-NSP2-1) as a high-quality chemical probe and (S)-1 (RA-NSP2-1N) as a negative control for probing the biology of alphavirus RNA helicases.