Glutamine transporters regulate prostate cancer radiosensitivity through NUPR1-mediated stress response
Glutamine transporters regulate prostate cancer radiosensitivity through NUPR1-mediated stress response
Kahya, U.; Lukiyanchuk, V.; Gorodetska, I.; Weigel, M. M.; Köseer, A. S.; Alkan, B.; Savic, D.; Linge, A.; Löck, S.; Peitzsch, M.; Skvortsova, I.-I.; Krause, M.; Dubrovska, A.
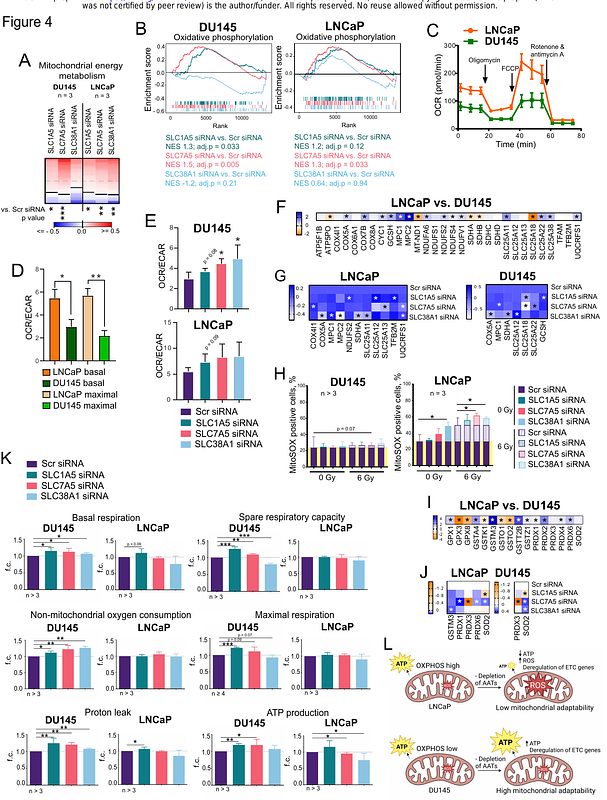
AbstractBackgroundMetabolic and stress response adaptations in prostate cancer (PCa) mediate tumor resistance to radiation therapy (RT). Our study investigated the roles of glutamine (Gln) transporters SLC1A5, SLC7A5, and SLC38A1 in regulating NUPR1-mediated stress response, PCa cell survival, metabolic reprogramming, and response to RT.\n\nMethodsThe radiosensitizing potential of GLS inhibition with CB-839 was analyzed in prostate cancer xenograft models. The level of gene expression was analyzed by RNA sequencing and RT-qPCR in the established cell lines or patient-derived tumor and adjacent non-cancerous tissues. Phosphoproteomic analysis was employed to identify the underlying signaling pathways. The publicly available PCa patient genesets, and a geneset for the patients treated with RT were analyzed by SUMO software. The key parameters of mitochondrial functions were measured by Seahorse analysis. Analysis of the general oxidative stress level and mitochondrial superoxide detection were conducted using flow cytometry. {gamma}H2A.X foci analysis was used to assess the DNA double strand break. Relative cell sensitivity to RT was evaluated by radiobiological clonogenic assays. Aldefluor assay and sphere-forming analysis were used to determine cancer stem cell (CSC) phenotype.\n\nResultsDepletion of these transporters led to reduced cell viability, altered ROS levels, and enhanced radiosensitivity in PCa cell lines. Functional assays revealed that targeting these transporters decreases CSC properties, impairs cell cycle progression, and deregulates mitochondrial energy metabolism. Our findings indicate that the Gln transporters mediate the adaptation of tumor cells to nutrient stress, while NUPR1 promotes cellular survival under metabolic and genotoxic stress. Targeting SLC1A5, SLC7A5, SLC38A1, and NUPR1 radiosensitizes PCa by disrupting metabolic adaptations and stress responses. Besides, CB-839, a glutaminase (GLS) inhibitor, combined with RT, demonstrated a synergistic effect with radiotherapy in vivo, significantly delaying tumor growth. We found that GLS gene expression levels are significantly associated with clinical outcomes in PCa patients treated with RT.\n\nConclusionsOur work underscores the role of Gln transporters and the NUPR1-mediated stress response induced by Gln deficiency in PCa cell survival, stemness, mitochondrial functions and radioresistance. Our findings provide a potential therapeutic in vivo strategy to enhance the efficacy of RT and improve treatment outcomes for PCa patients.