Mx1-Cre-mediated Rac1 knockout confers multiple protective effects against anthracycline-induced acute normal tissue injury
Mx1-Cre-mediated Rac1 knockout confers multiple protective effects against anthracycline-induced acute normal tissue injury
Kucuk, P.; Gatzmanga, S.; Aengenvoort, J.; Henninger, C.; Heinrich, C.; Vijayendran, A.; Fritz, G.
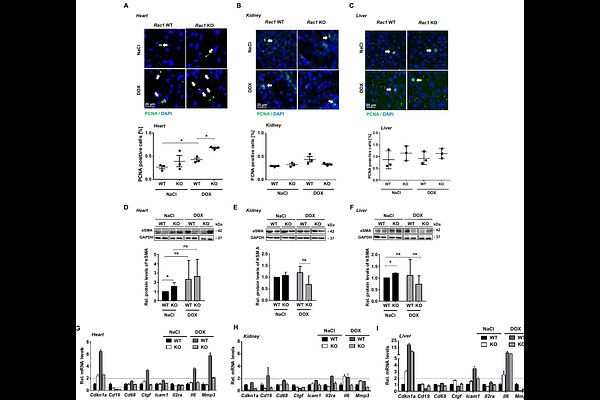
AbstractPharmacological data point to RAC1 as promising target for protection against anthracycline-induced cardiotoxicity, yet supporting genetic evidence is limited. Moreover, the relevance of RAC1 for cross-organ injury and cross-agent-induced normal tissue toxicity is unknown. Here, we employed a Mx1-Cre-based mouse model that enables an inducible Rac1 knockout across different organs in order to comparatively analyze the influence of RAC1 on doxorubicin (DOX) and cisplatin (CisPt)-induced acute stress responses of the heart, kidney and liver. Following DOX treatment, the extend of DNA-double strand break (DSB) formation and the percentage of apoptotic cells were reduced in all three organs (i.e. heart, liver, kidney) of RAC1 deficient (Rac1-/-) animals as compared to the wildtype control (Rac1+/+). By contrast, in the absence of RAC1, CisPt-induced DNA damage was reduced only in the liver and the frequency of CisPt-stimulated apoptotic cell death remained unaffected by the Rac1 status in all organs. These findings demonstrate a strikingly organ- and agent-specific relevance of RAC1-regulated mechanisms for normal tissue damage evoked by genotoxic anticancer therapeutics. Accordingly, protein levels of phosphorylated DNA damage response (DDR)-related factors were also reduced in the kidney and liver of DOX treated Rac1-/- animals, but not in the heart. Yet, DOX-triggered mRNA expression of surrogate markers related to inflammation, fibrosis and senescence was preferentially reduced in the heart and kidney of Rac1 deficient mice. Taken together, RAC1 plays a so far unknown distinct role in the pathophysiology of anticancer drug-induced normal tissue damage by influencing DNA damage formation, activation of DDR, apoptosis induction as well as inflammation-, fibrosis- and senescence-associated responses in a pronounced agent- and organ-specific manner. Targeting of RAC1 appears particularly effective in the context of anthracycline-based therapeutic regimen for conferring broad organoprotection to both the heart and detoxifying organs.