Divalent HIV-1 gp120 Immunogen Exhibits Selective Avidity for Broadly Neutralizing Antibody VRC01 Precursors
Divalent HIV-1 gp120 Immunogen Exhibits Selective Avidity for Broadly Neutralizing Antibody VRC01 Precursors
Bailey, R.; Kahoekapu, K.; To, A.; Mayerlen, L.; Kae, H.; Manninen, G.; Haun, B. K.; Berestecky, J.; Shikuma, C.; Lehrer, A. T.; MacPherson, I. S.
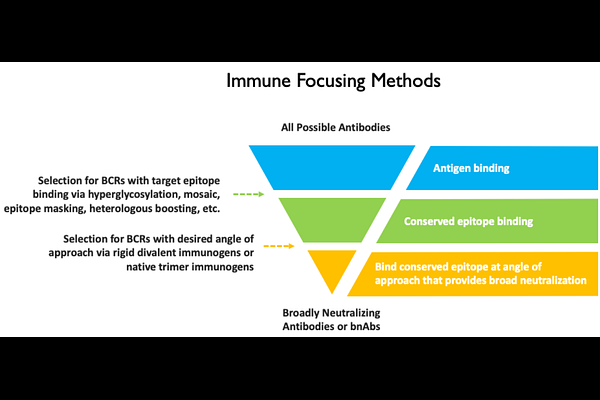
AbstractA major goal for the vaccine field is elicitation of broadly neutralizing antibodies (bnAbs) against pathogens that exhibit extensive antigenic diversity. In this study, we designed a rigid divalent immunogen for high avidity binding to the bnAb, VRC01, which targets the CD4 binding site (CD4bs) of HIV spike protein. This was accomplished by covalently linking two HIV-1 gp120 antigens to a complementary antibody and crosslinking the light chains. The divalent immunogen exhibits a higher affinity for VRC01-class antibodies compared to a non-Fab-Fab-crosslinked control, likely due to antigen pre-organization limiting the entropic penalty for divalent binding. Importantly, this immunogen exhibited divalent binding to VRC01 and monovalent binding to a non-CD4bs Ab, A32 - a characteristic we refer to as \"selective avidity.\" This report supports future in vivo vaccination experiments to test the immune focusing properties of this immunogen, the results of which may suggest broad application of the selective avidity concept.