The response of the skeletal muscle transcriptome to exercise in chronic kidney disease
The response of the skeletal muscle transcriptome to exercise in chronic kidney disease
Baker, L.; Graham-Brown, M.; Wilkinson, T.; Smith, A.; Watson, E.
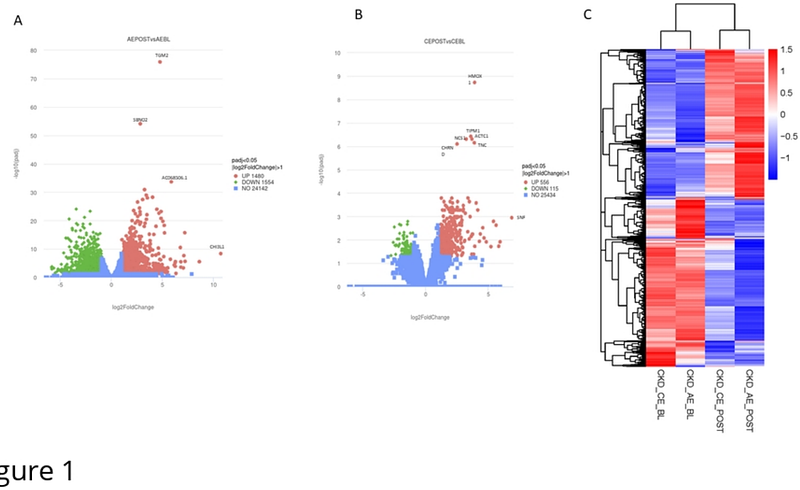
AbstractBackground Chronic kidney disease (CKD) affects approximately 14% of the UK population and is associated with significant exercise intolerance, partly due to skeletal muscle dysfunction. While exercise is a potential therapeutic strategy, the molecular response of skeletal muscle to exercise in CKD remains poorly understood. This study aimed to characterise transcriptomic changes in skeletal muscle 24 hours after aerobic (AE) or combined aerobic and resistance exercise (CE) in non-dialysis CKD. Methods This study utilised muscle biopsies from participants in the ExTRA CKD trial with stage 3b-4 CKDages 3b-4 (AE: 24 (15-32) ml/min/1.73m2; CE: 25 (19-31) ml/min/1.73m2). Participants (n=4 per group) were randomised to 12 weeks of thrice-weekly AE or CE. Vastus lateralis skeletal muscle biopsies were collected at baseline and 24h after the first bout of exercise. RNA was extracted for Bulk RNA sequencing. Bulk RNA sequencing was performed, and differentially expressed genes (DEGs) were identified between baseline and post-exercise samples, followed by pathway enrichment analysis. Results Following AE, 1480 genes were upregulated and 1554 downregulated. CE resulted in 556 upregulated and 115 downregulated genes. The most upregulated gene after AE was CHI3L1 (log2FC 10.7), followed by SAA2 and PTX3, all associated with inflammation. After CE, SFN (log2FC 6.8) and MT1A were among the most highly upregulated. Enrichment analysis showed strong activation of inflammatory and cellular senescence pathways, and downregulation of mitochondrial function-related processes, particularly after AE. Conclusion Both AE and CE triggered robust inflammatory gene expression responses in CKD skeletal muscle, indicative of early repair processes. Unexpectedly, mitochondrial-related pathways were downregulated, aligning with previous findings of impaired mitochondrial adaptation in CKD. These results highlight mitochondrial dysfunction as a potential barrier to effective exercise adaptation and a possible therapeutic target in this population.