Endothelial Kallikrein-Related Peptidase 8 Promotes Diabetic Nephropathy through a LIFR dependent mechanism
Endothelial Kallikrein-Related Peptidase 8 Promotes Diabetic Nephropathy through a LIFR dependent mechanism
Ni, X.; Du, J.; Li, M.; Jiang, Y.; Tang, Z.; Xu, D.-H.; Yin, H.; Yuan, J.; Zhu, X.-Y.
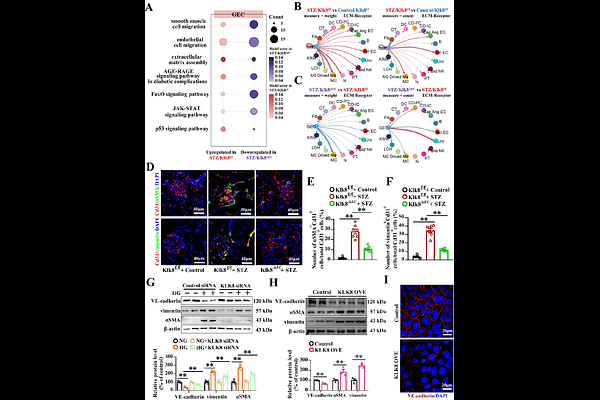
AbstractBackground: Diabetic nephropathy (DN) is the primary microvascular complication of diabetes mellitus; however, the exact pathways in endothelial cells (ECs) linked to DN progress remain unclear. Tissue kallikrein-related peptidases (KLKs) participate in pathophysiological processes in ECs. We aimed to explore the roles of endothelial KLKs in DN and define the underlying mechanisms. Methods and results: KLK8 was the most highly upregulated member of KLKs in renal tissues in streptozotocin (STZ)-induced diabetic mice and cultured glomerular ECs (GECs) upon high glucose (HG) treatment. Both global (Klk8-/-) and endothelial Klk8 knockout (Klk8EC) mice displayed improved albuminuria, mesangial matrix expansion and glomerulosclerosis caused by STZ compared to Klk8f/f mice. Single-cell RNA seq (scRNA-seq) showed that many pathways associated with DN in ECs, mesangial cells (MCs) and tubule cells were reversed in Klk8EC mice. Endothelial-to-mesenchymal transition (EndMT) was extensively improved in Klk8EC mice, and by KLK8 siRNA in cultured GECs upon HG. Using proteome and other biochemical approaches, we revealed that KLK8 cleaved syndecan-4(SDC4), which contributed to loss of glycocalyx integrity in GECs in cultured cells and animal diabetic models. Furthermore, scRNA-seq showed that Lifr was one of the key genes linked to the disease progressed in ECs and MCs and regulated by endothelial Klk8. LIFR signaling contributed to HG-induced GEC dysfunction and MC activation, which was associated with endothelial Klk8. Knockdown of Lifr by lentivirus-Lifr shRNA ameliorated hallmark features of DN and improved EndMT and Sdc4 expression glomeruli of diabetic mice. LIFR was upregulated in GECs and MCs in DN patients. Circulatory levels of LIF, KLK8 and soluble SDC4 were increased in patients with DN, and KLK8 level was positively correlated with LIF, soluble SDC4 and creatinine levels. Conclusion: Endothelial KLK8 promotes GECs dysfunction and abnormal crosstalk with MCs through a LIFR-dependent signaling in DN, which immediately highlights therapeutic targets for DN.