Splicing variants in MYRF cause partial loss of function in the retinal pigment epithelium
Splicing variants in MYRF cause partial loss of function in the retinal pigment epithelium
Rozumek, G. M.; Brinkmeier, M. L.; Guan, B.; Wang, S. Q.; Tower, C.; Yang, N. T.; Lim, R.; Dong, L.; Hannum, D. F.; Moroi, S. E.; Richards, J. E.; Hufnagel, R. B.; Prasov, L.
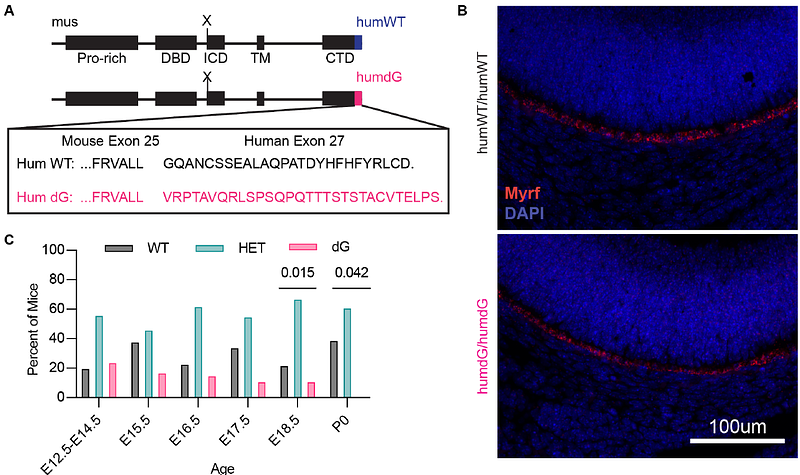
AbstractMyelin Regulatory Factor (MYRF) regulates retinal pigment epithelial (RPE) development and variants in the C-terminus are linked to isolated nanophthalmos, while loss-of-function variants cause syndromic disease. To define the molecular mechanism of this discrepancy, in vitro and animal studies were performed on a pathogenic C-terminal variant (p.Gly1126fs30* or dG-MYRF). ARPE-19 cells transduced with dG-MYRF revealed reduced target gene expression compared to WT-MYRF, with reduced steady state levels of C-terminal MYRF cleavage product, but intact cleavage and localization. A homozygous humanized MYRF C-terminal (MyrfhumdG/humdG) mouse model was embryonic lethal by embryonic day (E) 18.5, while humanized wildtype (MyrfhumWT/humWT) showed normal expression and survival. Bioinformatic analysis on integrated single cell RNA-seq from humanized E17.5 and knockout Rx-Cre;Myrffl/fl (E15.5 and P0) mice supported shared differentially expressed genes with decreased effect size in MyrfhumdG/humdG eyes. These findings, and the viability differences, support that dG-MYRF is a hypomorphic allele. Further, two novel MYRF splicing variants were identified in families with isolated nanophthalmos, with one confirmed to alter 40% of spliced transcripts, creating a nonfunctional isoform. These cases corroborate that isolated nanophthalmos results from hypomorphic alleles of MYRF, supporting a tissue-specific threshold effect and suggests that the C-terminus has unique roles in the RPE.