Replication of SARS-CoV-2 Omicron lineages is defined by TMPRSS2 use in environments where ACE2 is complexed with solute carriers SLC6A19 and SLC6A20.
Replication of SARS-CoV-2 Omicron lineages is defined by TMPRSS2 use in environments where ACE2 is complexed with solute carriers SLC6A19 and SLC6A20.
Aggarwal, A.; Ognenovska, S.; Ison, T.; Fichter, C.; Milogiannakis, V.; Afzal, M.; Ospina Stella, A.; Waters, S.; Esneau, C.; Bartlett, N.; Pöhlmann, S.; Hoffmann, M.; Burrell, L. M.; Patel, S.; Ellis, S.; Wehrhahn, M.; Ginn, A.; Martinez, E.; Churchill, M.; Angelovich, T.; Rawlinson, W. D.; Yeang, M.; Kok, J.; Sintchenkov, V.; Parry, R.; Sng, J. D.; Neely, G. G.; Moreno, C. L.; Loo, L.; Kelleher, A. D.; Brilot, F.; Khromykh, A.; Turville, S. G.
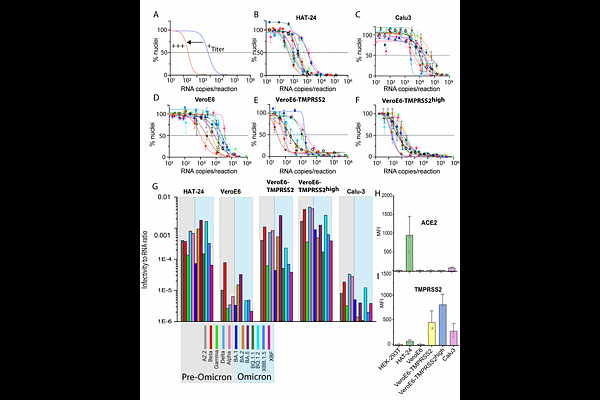
AbstractThe Omicron variant of SARS-CoV-2 emerged in late 2021 and since then Omicron subvariants have continued to evolve and dominate globally. The viral S protein evolved towards highly efficient antibody evasion and replicative capacity in the upper respiratory tract resulting in high transmissibility. At the same time, the mutations acquired in the S protein diminish infection of the lung epithelium and pathogenic potential. The changing entry requirements for Omicron sub-lineages that lead to this shift in tropism remain poorly understood. We resolve the changing replication requirements of SARS-CoV-2 to be related to two distinct pools of ACE2. The first pool relates to ACE2s role in the renin angiotensin system (RAS) and this pool can complex with TMPRSS2 (RAS-ACE2). The second pool relates to ACE2s role as a protein solute carrier chaperone than cannot complex with TMPRSS2 (Chaperone ACE2). Here, we demonstrate that pre-Omicron lineages replicate in a TMPRSS2 dependent manner across both ACE2 pools, whilst Omicron lineages can only spread and replicate using chaperone ACE2. This provides a mechanistic basis for the evolving infectivity requirements of SARS-CoV-2 and furthermore provides approaches to track and monitor ACE2 utilizing coronaviruses.