Transient Formation of Supramolecular Complexes Between Hyaluronan and Oligopeptides at Submicromolar Concentration
Transient Formation of Supramolecular Complexes Between Hyaluronan and Oligopeptides at Submicromolar Concentration
Riopedre-Fernandez, M.; Chu, B.; Kuffel, A.; Marchioro, A.; Biriukov, D.; Martinez-Seara, H.
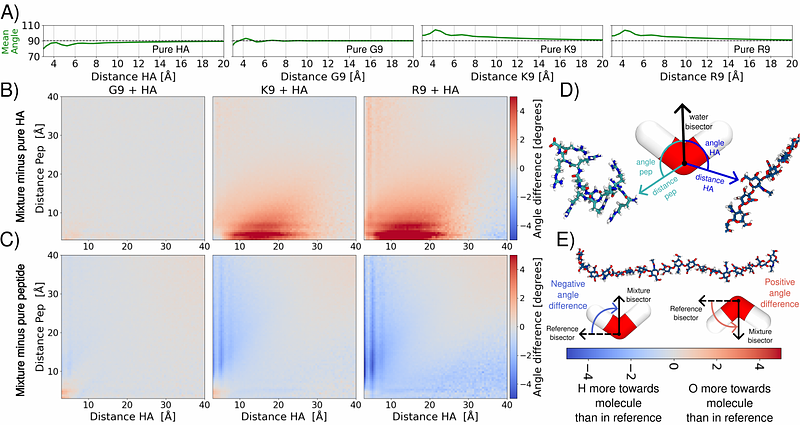
AbstractCharged polymer interactions govern critical biological and technological processes by altering the structure and dynamics of the surrounding aqueous environment. However, studying these interactions and the resulting environments across a broad concentration range is challenging, as it demands a combination of multiple techniques with varying resolutions and complex data interpretation. In particular, detecting interactions at submicromolar levels remains technically challenging, with most methods lacking the resolution required for molecular-level characterization. Here, we examined the interactions between high-molecular-weight hyaluronan (HA), a biologically and technologically relevant polymer, and a series of model oligopeptides--nonaarginine, nonalysine, and nonaglycine, chosen for their charge and side-chain chemistry. Using angle-resolved second harmonic scattering (AR-SHS), multi-angle dynamic light scattering, nuclear magnetic resonance spectroscopy, and all-atom molecular dynamics simulations, we captured a detailed molecular picture of HA-peptide interactions across a broad concentration range, including submicromolar concentrations. We found selective and multivalent interactions between HA and positively charged peptides, which are consistently stronger with nonaarginine. These interactions trigger significant solvent and solute remodeling, including nanoscale HA-peptide clustering. Our molecular simulations provide essential atomic-level interpretation of the experimental data, elucidating the transient and dynamic nature of the intermolecular interactions and the underlying processes of molecular aggregation and induced water reorientation. The distinct behavior found in arginine-rich peptides highlights their potential in modulating extracellular environments and as peptide-based drug delivery systems. Moreover, our methodological framework, combining sensitive AR-SHS signals with atomistic simulations and traditional structural techniques, offers unprecedented molecular insight into complex polymer-peptide interactions, laying the groundwork for future research on dynamic supramolecular systems in soft materials and unstructured biological environments such as the extracellular matrix.