Alterations in Electroencephalography Signals in Female Fragile X Syndrome Mouse Model on a C57Bl/6J Background
Alterations in Electroencephalography Signals in Female Fragile X Syndrome Mouse Model on a C57Bl/6J Background
Wang, B.; Ahmed, A.; Murari, K.; Cheng, N.
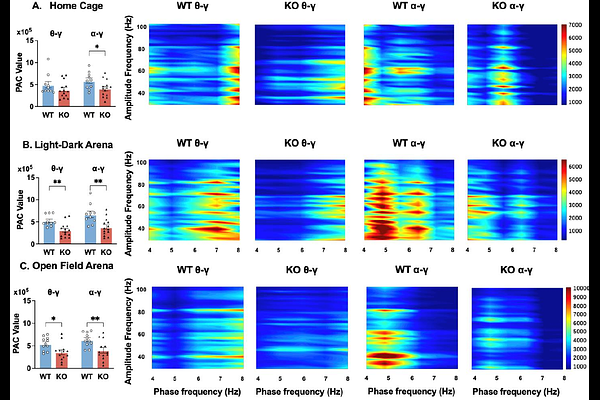
AbstractBackground: Fragile X Syndrome (FXS), the most common monogenic cause of autism spectrum disorder, arises from FMR1 gene silencing and exhibits pronounced sex differences in prevalence and phenotypic severity. Electroencephalography (EEG) has emerged as a promising translational biomarker for FXS pathophysiology, yet prior research has predominantly focused on male cohorts. In the widely used C57Bl/6J (B6) mouse strain, male Fmr1 knockout (KO) models show increased absolute gamma power at both juvenile and adult stages, which may reflect cortical hyperexcitability. In contrast, little is known about female Fmr1 KO mice, except that they exhibit no gamma alterations in adulthood. This gap hinders understanding of sex-specific neurodevelopmental trajectories of EEG profile in FXS. Leveraging the genetic stability and translational relevance of the B6 strain, this study compares EEG profiles between juvenile female Fmr1 KO and wild-type (WT) B6 mice to address this critical gap. Methods: Frontal-parietal differential EEG was recorded in freely behaving mice using the Open-Source Electrophysiology Recording system for Rodents. Neural activity was analyzed across three recording conditions: in the home cage, light-dark arena, and open field arena. Computed metrics included absolute/relative power, peak alpha frequency, theta-beta ratio, phase-amplitude coupling, amplitude-amplitude coupling, and multiscale entropy to assess signal complexity. Results: In all recording conditions, Fmr1 KO mice exhibited reduced absolute power in theta, alpha, and beta frequency bands compared to WT controls. Relative power analysis revealed decreased alpha activity alongside increased gamma-band power, including both low and high gamma, in the KO mice. Cross-frequency coupling was disrupted, with diminished alpha-gamma phase-amplitude coupling. Amplitude-amplitude coupling between theta or alpha and gamma power displayed distinct changes in different recording conditions. Peak alpha frequency and theta-beta ratio were both reduced or unchanged in the KO mice, depending on the recording condition. Finally, EEG signal complexity remained comparable between the two genotypes across the conditions. Behaviorally, KO mice displayed hyper-exploration in the open field test, characterized by increased center time and entries. However, no overall robust correlations between EEG power in different frequency bands and behavioral parameters in the open field test were observed. Discussion and Conclusion: Our results demonstrate that juvenile female Fmr1 KO mice on a B6 background exhibit EEG alterations highly consistent with those reported in FXS patients, particularly increased gamma and reduced alpha power. The robust increase in gamma activity reinforces its status as a reliable biomarker across preclinical and clinical studies, while alpha reductions and slowed peak alpha frequency implicate thalamocortical network involvement. Together, these findings highlight the translational value of this model for studying core circuit dysfunctions in FXS.