Collaboration between two conserved sequence motifs drives ATPase stimulation of Hsp90 by Aha1
Collaboration between two conserved sequence motifs drives ATPase stimulation of Hsp90 by Aha1
Amoah, D. P.; Mercier, R.; Alyousef, G.; Johnson, J. L.; LaPointe, P.
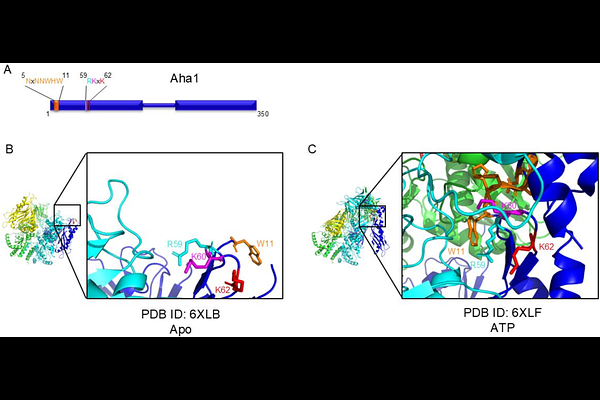
AbstractHsp90 is a dimeric molecular chaperone essential for the folding, stabilization, activation, and maturation of hundreds of client proteins, which are critical for cellular function. Co-chaperones, such as Aha1, play a key role in regulating the ATP-dependent Hsp90 client activation cycle by modulating Hsp90\'s ATPase activity and controlling progression through the cycle. Two highly conserved motifs in Aha1-the NxNNWHW and RKxK motifs-are known to regulate specific aspects of the Hsp90 ATPase cycle. In this study, we demonstrate that the K60 residue within the RKxK motif facilitates the structural organization of the NxNNWHW motif prior to ATP hydrolysis. Mutation of the K60 residue partially impairs the in vivo functionality of yeast Aha1. Additionally, we reveal that each individual residue within the NxNNWHW motif modulates the ATPase rate and apparent affinity for ATP of Hsp90. These findings provide new insights into how conserved regions of Aha-type co-chaperones influence Hsp90 kinetics and its regulation of client protein folding.