The pleuroparenchymal fibroelastosis atlas reveals aberrant cell states and their zonation as an alternate roadmap to lung fibrosis
The pleuroparenchymal fibroelastosis atlas reveals aberrant cell states and their zonation as an alternate roadmap to lung fibrosis
Ruwisch, J.; Cazes, A.; Leiber, L. M.; Borie, R.; Neubert, L.; Christian, L.; De Montpreville, V. T.; Szmul, A.; Moussa, F.; Verleden, S.; Gaedcke, S.; Hegermann, J.; Fuge, J.; Ballmaier, M.; Kamp, J. C.; Greer, M.; Braubach, P.; Werlein, C.; Ius, F.; Graalmann, T.; Aburahma, K.; de Sadaleer, L.; Egashira, R.; Ackermann, M.; Yamda, D.; Hoeper, M.; Falk, C.; Gottlieb, J.; Schiller, H.; Vanaudenaerde, B.; Seeliger, B.; Debray, M.-P.; Bernaudin, J. F.; Knudsen, L.; Bergot, E.; Jacob, J.; Mal, H.; Jonigk, D.; Dettmer, S.; Mordant, P.; Prasse, A.; Fadel, E.; Wuyts, W.; Crestani, B.; Kaminski, N.; J
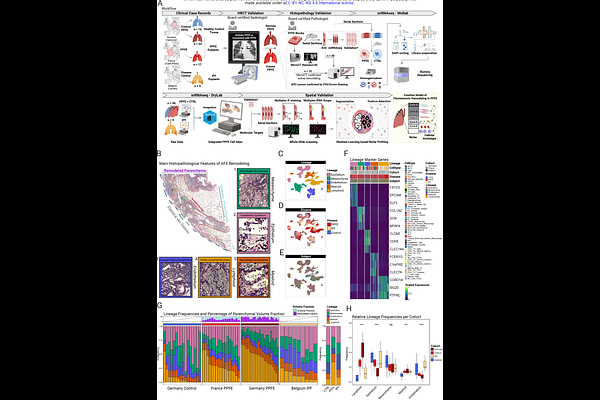
AbstractBackground: Pleuroparenchymal fibroelastosis (PPFE) is a progressive interstitial lung disease with higher prevalence in females, histologically characterized by intra-alveolar fibrosis with septal elastosis (AFE). Effective treatments are lacking, highlighting the need to dissect its pathogenesis at single-cell resolution. Methods: We performed single-nucleus RNA sequencing (snRNAseq) on explanted lungs from a German (n=23) and a French cohort (n=17) of PPFE patients, and controls (n=16). Identified cell populations were localized by immunofluorescence and multiplex RNA in-situ hybridization. Hierarchical phase-contrast computed tomography (HiP-CT) and micro-CT provided 3D spatial context. Reanalyzed snRNAseq data from a Belgian IPF cohort (n=9) served as disease comparator. Findings: We present the first snRNAseq atlas of PPFE\'s cellular and structural landscape based on a European multinational cohort. 24 PPFE patients were female (60.0%), while 34 were non-smokers (85.0%). 519,920 nuclear transcriptomes from PPFE and IPF patients, and controls were profiled. We identified PPFE-specific accumulations of MFAP5+PI16+SFRP2+ adventitial and LEPR+ITGA8+SFRP2+DIO2+ elastofibrotic fibroblasts as main drivers of elastotic remodeling in PPFE. Multiple PPFE fibroblast subsets acquire an inflammatory activation state as highlighted by the expression of CXCL12 and CXCL14. This is accompanied by a marked increase in lymphocytes and the formation of tertiary lymphoid structures (TLS) in a disease that was previously considered to be purely elastofibrotic. We identified CTHRC1+ fibrotic fibroblasts and Aberrant Basaloid cells in PPFE as well, forming the \'Usual Fibrotic Niche\'. 3D reconstruction of the pronounced COL15A1+ vascular conglomerate at the border of the elastofibrotic and subpleural fibrosis indicates communication with interlobar veins. Last, we observed a zonation of the PPFE lesion, constructed by the above-mentioned PPFE-associated cell types. Interpretation: Our unprecedented cellular and molecular survey uncovers previously unobserved PPFE-specific inflammatory and elastogenic fibroblast populations, as well as the presence of CTHRC1+ fibroblasts and Aberrant Basaloid cells common to other fibrotic ILDs. These findings provide the foundation for including PPFE patients in current antifibrotic trials, as well as development of PPFE-specific therapies.