Single-nucleus and spatial transcriptomic profiling of human temporal cortex and white matter reveals novel associations with AD pathology
Single-nucleus and spatial transcriptomic profiling of human temporal cortex and white matter reveals novel associations with AD pathology
Gaur, P.; Bryois, J.; Calini, D.; Foo, L.; Hoozemans, J. J. M.; Malhotra, D.; Menon, V.
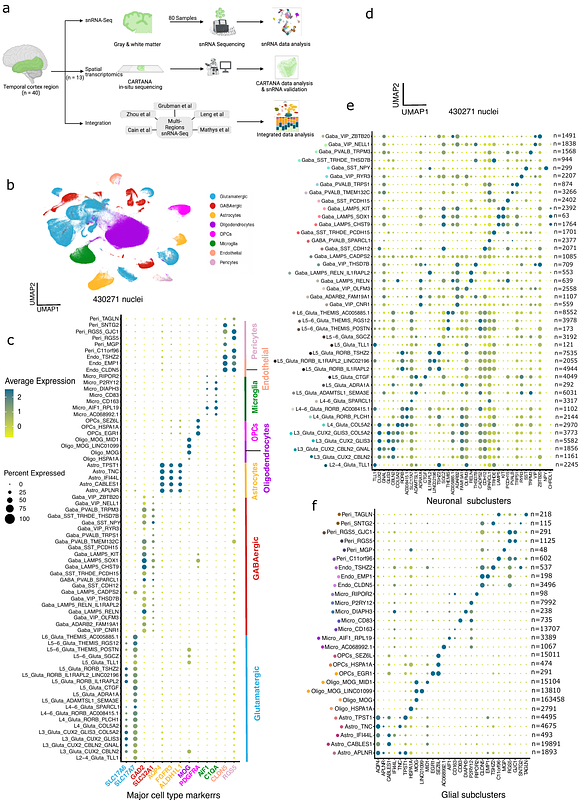
AbstractAlzheimer\'s disease (AD) is a neurodegenerative disorder with complex pathological manifestations and is the leading cause of cognitive decline and dementia in elderly individuals. A major goal in AD research is to identify new therapeutic pathways by studying the molecular and cellular changes in the disease, either downstream or upstream of the pathological hallmarks. In this study, we present a comprehensive investigation of cellular heterogeneity from the temporal cortex region of 40 individuals, comprising healthy donors and individuals with differing tau and amyloid burden. Using single-nucleus transcriptome analysis of 430,271 nuclei from both gray and white matter of these individuals, we identified cell type-specific subclusters in both neuronal and glial cell types with varying degrees of association with AD pathology. In particular, these associations are present in layer specific glutamatergic (excitatory) neuronal types, along with GABAergic (inhibitory) neurons and glial subtypes. These associations were observed in early as well as late pathological progression. We extended this analysis by performing multiplexed in situ hybridization using the CARTANA platform, capturing 155 genes in 13 individuals with varying levels of tau pathology. By modeling the spatial distribution of these genes and their associations with the pathology, we not only replicated key findings from our snRNA data analysis, but also identified a set of cell type-specific genes that show selective enrichment or depletion near pathological inclusions. Together, our findings allow us to prioritize specific cell types and pathways for targeted interventions at various stages of pathological progression in AD.
