Structural and mechanistic analysis of covalent ligands targeting the RNA-binding protein NONO
Structural and mechanistic analysis of covalent ligands targeting the RNA-binding protein NONO
Lindsey, G. L.; Hockley, T. K.; Gomez, A. V.; Marshall, A. C.; Brothers, W. R.; Finney, C. T.; Gross, J.; Fox, A. C.; Yeo, G. W.; Melillo, B.; Bond, C. S. S.; Cravatt, B. F.
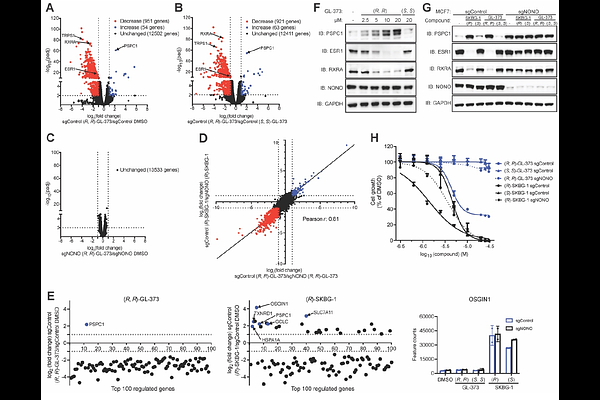
AbstractRNA-binding proteins (RBPs) play important roles in mRNA transcription, processing, and translation. Chemical tools are lacking for RBPs, which has hindered efforts to perturb and understand RBP function in cells. We recently reported a chloroacetamide compound (R)-SKBG-1 that covalently binds the RBP NONO and stabilizes its interactions with mRNAs, leading to transcriptional remodeling and the suppression of cancer cell growth. Here, we report the crystal structure of an (R)-SKBG-1:NONO complex, which confirms covalent modification of cysteine-145 at a pocket proximal to the RNA-binding interface of the protein. We show that this pocket can also be targeted by a lower reactivity chlorofluoroacetamide analog (R, R)-GL-373, which retains the pharmacological properties of (R)-SKBG-1, including blockade of estrogen receptor expression in breast cancer cells, while displaying much greater proteome-wide selectivity. Our findings thus show that NONO can be targeted by covalent ligands with high specificity to pharmacologically suppress pro-tumorigenic gene products in cancer cells.