Global analysis of membrane protein S-acylation in the model plant Arabidopsis thaliana
Global analysis of membrane protein S-acylation in the model plant Arabidopsis thaliana
Zhou, L.; Su, L.; Zhou, M.; Gritsenko, M. A.; Wan, J.; Ma, Y.; Zhao, Y.; Pasa-Tolic, L.; Xu, D.; Stacey, G.
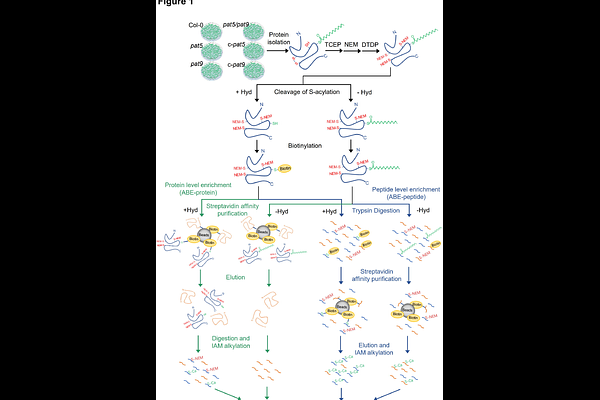
AbstractProtein S-acylation is the addition of fatty acids to the cysteine residues in a protein, catalyzed by protein S-acyltransferases (PATs). Despite extensive research on protein S-acylation in animals, our understanding of this process in plants remains limited. In this study, we sought to characterize the S-acylproteome of membrane proteins in Arabidopsis and identify potential substrates for two important plant immunity-related PATs (PAT5 and PAT9). To achieve this, S-acylated membrane proteins were first enriched via our optimized acyl-biotinyl exchange strategies at both the protein-level and peptide-level. The enriched samples were then analyzed by label-free quantitative liquid chromatography-mass spectrometry. The results from the two enrichment methods demonstrated that they were complementary in identifying S-acylated proteins and S-acylation sites. Using these methods, over 2500 S-acylation sites in more than 2000 putative S-acylated proteins were identified. Proteins involved in vesicle trafficking, plant phosphorylation, immune responses, and signal transduction pathways were significantly enriched. Additionally, certain amino acid patterns surrounding the S-acylation sites were identified. Comparisons of the S-acylproteomes between the wild type and the PAT5 and PAT9 mutants revealed over 100 potential substrates for both S-acyltransferases. The high quality of our data was supported by the significant overlap with the previously reported data and successful experimental verification of selected candidate proteins. Overall, our study revealed a well-represented S-acylproteome for Arabidopsis (especially its membrane proteins) and identified potential substrates for PAT5 and PAT9. These findings will facilitate the functional characterization of S-acylated proteins in plants.