TCA cycle rewiring underpins implantation and histone acetylation programming
TCA cycle rewiring underpins implantation and histone acetylation programming
Kafkia, E.; Pladevall-Morera, D.; Argemi-Muntadas, L.; Wang, G.; Noberini, R.; Bages-Arnal, S.; Anagho-Mattanovich, M.; Silverio-Alves, R.; Bonaldi, T.; Rabelink, T. J. J.; Moritz, T.; Zylicz, J. J.
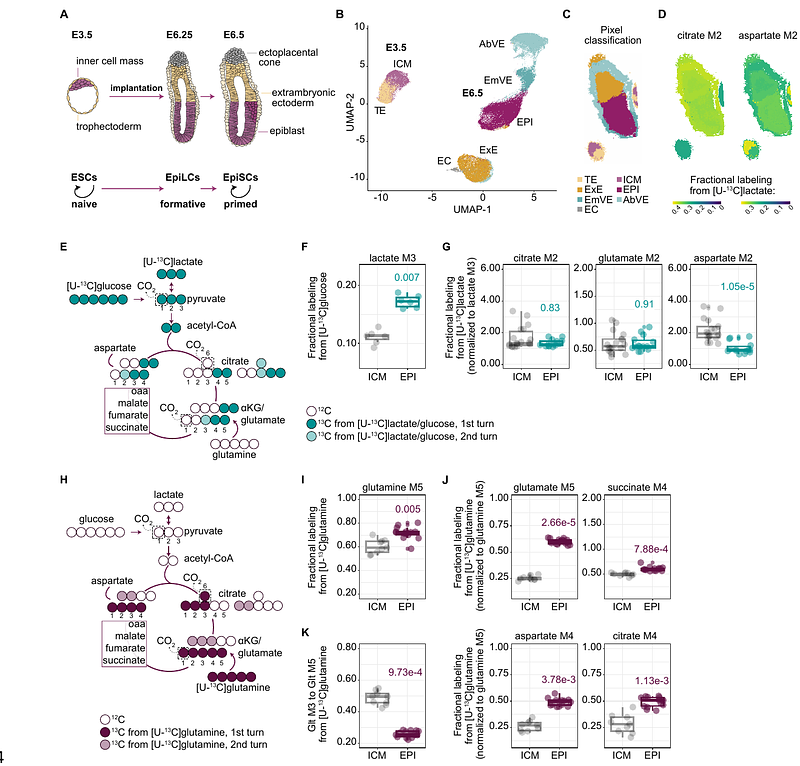
AbstractMetabolism has emerged as a key regulator of stem cell differentiation and their epigenomes. This coupling is particularly evident during the exit from naive pluripotency in vitro. However, our understanding of the dynamics of the metabolic rewiring especially at implantation remains rudimentary. In this study, we reconstruct the intracellular metabolite routings in pre- and post-implantation mouse embryos and during dynamic pluripotency transitions of cultured stem cells. Our findings reveal that, instead of a simple TCA cycle shutdown, there is a spatio-temporally programmed rewiring of the TCA cycle at implantation. Focusing on the spectrum of pluripotent cells, we identify pyruvate as a key metabolic nexus. Indeed, pyruvate carboxylase and malic enzyme activity establish cyclical carbon flow, which is essential for maintaining a balanced metabolic and transcriptional state and timely exit from naive pluripotency. Additionally, we discover that formative and primed pluripotent cells exhibit increased glutamine contribution to the TCA cycle, reduced oxidative TCA activity, and reciprocal reductive glutamine metabolism. This metabolic rewiring supports increased histone acetylation turnover, primarily using glutamine as a carbon source, supplemented by pyruvate cycling. Thus, we uncover diverse nutrient strategies that are functionally coupled to epigenome programming and dynamic pluripotency cell state transitions at the time of implantation.