Experience-dependent sharp-wave ripple deficits in an Alzheimer's disease mouse model
Experience-dependent sharp-wave ripple deficits in an Alzheimer's disease mouse model
Schnur, P.; Byron, N.; Gerasimou, S.; Pascual Clavel, A.; Rohner, V.; Patriarchi, T.; Sakata, S.
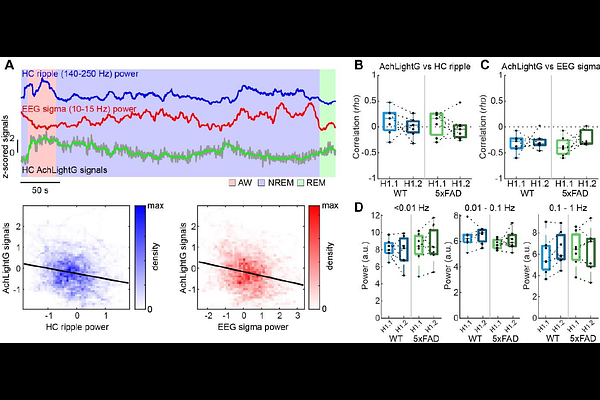
AbstractAmyloid pathology is a hallmark of Alzheimer\'s disease (AD). Hippocampal sharp-wave ripples (SWRs) play a role in memory consolidation and are impaired in various AD mouse models. However, it remains unclear how experience affects SWRs and how extrinsic signals contribute to SWR generation in AD. Here, by combining behavioral, in vivo electrophysiological and fiber photometry approaches, we show that an experience-dependent increase in hippocampal SWRs is disrupted in male and female 5xFAD mice. In wild-type mice, SWRs during non-rapid eye movement sleep (NREMS) increased after exploring a novel environment and the SWR rate gradually decreased with NREMS episodes and multiple behavioral sessions. However, 5xFAD mice did not show such experience-dependent SWR rate changes. A similar deficit was observed after a novel object recognition test. On the other hand, sleep spindles were intact in 5xFAD mice under all conditions. Since deficits in basal forebrain cholinergic neurons have been implicated in 5xFAD mice and SWRs are regulated by hippocampal cholinergic tone, we examined if hippocampal cholinergic signals could explain experience-dependent SWR deficits. Using fiber photometry and expressing a genetically encoded acetylcholine (ACh) sensor in the hippocampus, we found that ACh dynamics in the hippocampus were intact in 5xFAD mice across sleep-wake cycles, including NREMS, while we also found a negative correlation of infraslow cortical signal power dynamics with hippocampal ACh signals during NREMS regardless of genotypes. These results suggest that experience-dependent SWR deficits stem from non-cholinergic pathological changes.