A Novel Model for Proton Transport Mediated by Uncoupling Protein 1
A Novel Model for Proton Transport Mediated by Uncoupling Protein 1
Jacobsen, L.; Menon, S.; Gaudry, M. J.; Hakami Zanjani, A. A.; Reinholdt, P.; Jastroch, M.; Khandelia, H.
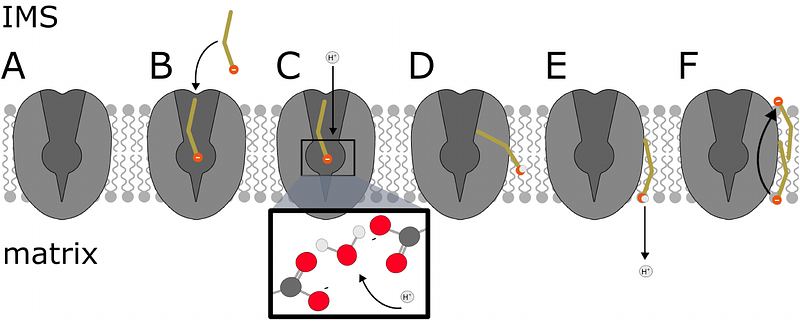
AbstractUncoupling Protein 1 (UCP1) is a mitochondrial protein which drives thermogenesis in brown adipose tissue. UCP1 facilitates the dissipation of the proton gradient as heat and plays a critical role in energy expenditure and metabolic regulation. We employ advanced molecular simulations and mutagenesis to reveal the mechanism of UCP1-mediated proton and fatty acid (FA) transport. We demonstrate that FAs bind spontaneously to UCP1\'s central substrate-binding site. In the binding site, a proton transfer to the FA is facilitated by a key aspartate residue (D28) and a coordinating water molecule. The protonated FA exits UCP1 through a well defined pathway, and releases its proton into the mitochondrial matrix. UCP1 then facilitates the return of deprotonated FAs to the intermembrane space. Nucleotide binding disrupts this mechanism by inducing conformational changes in the transmembrane helices and obstructing the FA return pathway. Our mechanism explains every step of the transport cycle, is supported by simulation and biochemical data, and explains a diverse set of biochemical data about the transport mechanisms in UCP1 and its analogues: ANT, UCP2 and UCP3.