Mitotic phosphorylation of Lamin B1 rod domain by ULK1 and Aurora A/PLK1 promotes spindle function.
Mitotic phosphorylation of Lamin B1 rod domain by ULK1 and Aurora A/PLK1 promotes spindle function.
Mendiburo, M. J.; Kalus, C.; Berleth, N.; Berning, L.; Sun, Y.; Hu, Z.; Shahba, A.; Naren, P.; Kasof, G.; Dengjel, J.; Stork, B.
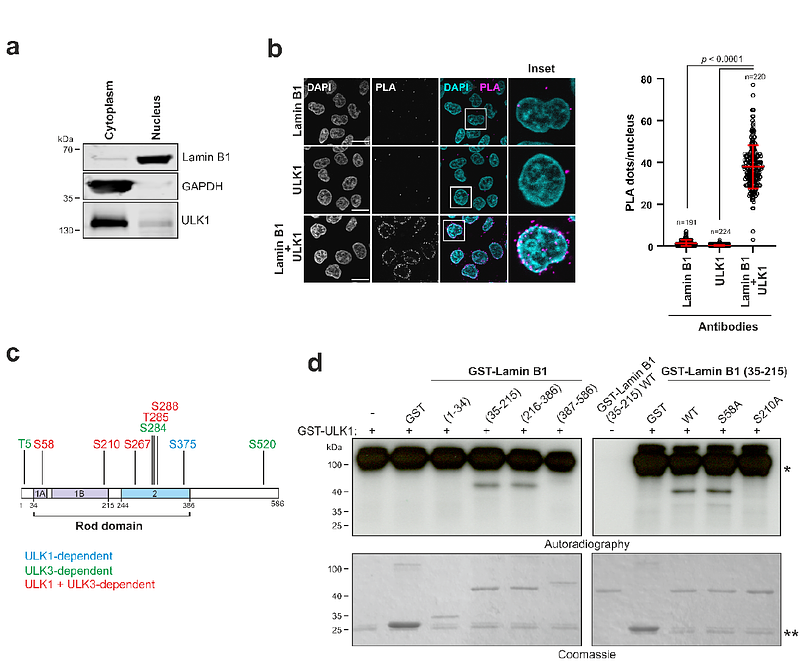
AbstractThe coil-coil rod domain mediating the lateral assembly of lamin filaments has been reported to undergo phosphorylation by proteomic approaches, but the functional implications of these modifications are currently unknown. Here, we report that serine 210 (S210) within the Lamin B1 rod domain is a mitotic phospho-acceptor residue controlled by the combined activities of the autophagy-activating kinase ULK1 and the mitotic kinases Aurora A and PLK1. The detection of Lamin B1 phospho-S210 with a specific phospho-antibody revealed its enrichment at the mitotic spindle and its association with a network of proteins with known functions in spindle assembly. Abrogation of the phosphorylation of the S210A Lamin B1 variant leads to an increase in the number of cells with multipolar or shorter spindles. We propose that mitotic phosphorylation of Lamin B1 S210 within the rod domain contributes to the maintenance of proper spindle function.