Structural Basis for a Scaffolding Role of the COM Domain in Nonribosomal Peptide Synthetases
Structural Basis for a Scaffolding Role of the COM Domain in Nonribosomal Peptide Synthetases
Diecker, J.; Hermanns, B.; Rueschenbaum, J.; Rasche, R.; Doerner, W.; Schroeder, A.; Kuemmel, D.; Mootz, H. D.
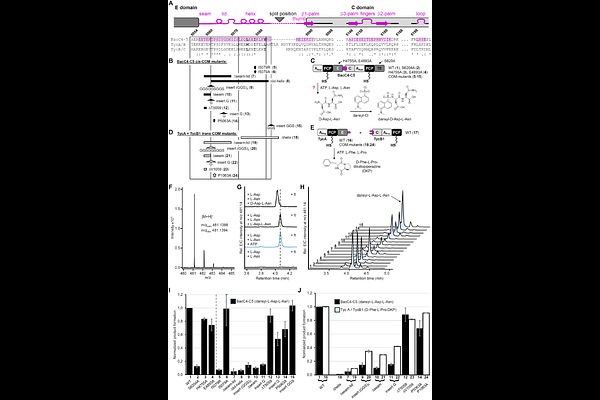
AbstractNonribosomal peptide synthetases (NRPSs) are multi-domain enzymes that catalyze the biosynthesis of therapeutically relevant natural products. Efficient peptide synthesis relies on intricate domain interactions, whose underlying principles remain poorly understood. The communication-mediating (COM) domains facilitate interactions between separate NRPS subunits like other docking domains, however, exhibit distinctive features that are unusual within this family: COM domains co-occur with epimerization (E) domains, are partially embedded within the adjacent condensation (C) domain and can also be found as an internal cis-COM domain with unknown function. We present the first crystal structure of a cis-COM domain within an E-COM-C domain arrangement from modules 4 and 5 of bacitracin synthetase 3 (BacC). The structure reveals a compactly folded COM domain sandwiched between E and C domains, suggesting a role in orienting these domains for efficient peptidyl carrier protein (PCP) shuttling. Through mutational analyses, dipeptide formation assays, and proximity-dependent photo-crosslinking experiments, we investigated both cis- and trans-COM domains and provide evidence supporting a principal role of COM domains as scaffolds of NRPS architecture. Their function as docking domains may be a secondary consequence of their division into separate donor and acceptor parts.