Spatial mapping of dextran sodium sulphate-induced intestinal inflammation and its systemic effects
Spatial mapping of dextran sodium sulphate-induced intestinal inflammation and its systemic effects
Adams, L.; Rasid, O.; Hulme, H.; Quon, T.; Burchmore, R.; Milling, S.; Goodwin, R.; Wall, D. M.
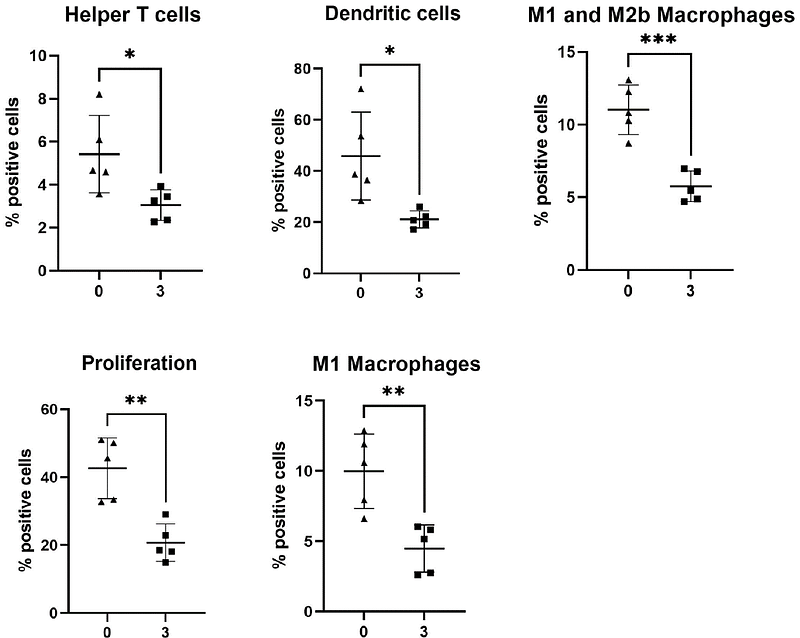
AbstractInflammatory bowel disease (IBD) is a multifactorial disease and patients frequently experience extraintestinal manifestations affecting multiple sites. Causes of systemic inflammation remain poorly understood but molecules originating from the intestine likely play a role with microbial and host small molecules polarizing host immune cells towards a pro- or anti-inflammatory phenotype. Using the dextran sodium sulphate (DSS) mouse model, which mimics models the disrupted barrier function in IBD, microbial dysbiosis and immune cell dysregulation in IBD, we investigated metabolomic and phenotypic changes at intestinal and systemic sites. Through mass spectrometry imaging we mapped the spatial distribution and relative abundance of molecules and cell types across a range of tissues during colitis. This approach revealed specific molecular changes across a range of organs including the colon, ileum, liver, spleen and kidney, while no molecular changes were observed in the lungs of DSS-treated mice. Specific molecules, identified as contributing to the statistical separation of treated from control mice, were then spatially localized within organs to determine their effects on cellular phenotypes through imaging mass cytometry. Additionally, molecules that were significantly changed across multiple systemic sites in response to inflammation were identified. This spatial approach identified drivers of inflammation both locally in the intestine and systemically and has highlighted a number of molecules not previously implicated in inflammation linked to IBD or the systemic effects of intestinal inflammation. Together this data shows that gaining a better understanding of metabolic pathways and identifying molecular disease biomarkers within the intestine and systemic organs during IBD, might improve our understanding of disease aetiology and aid the development of new targeted therapies.
