Cholinergic Signaling Modulates Intestinal Pathophysiology in a Drosophila Model of Cystic Fibrosis
Cholinergic Signaling Modulates Intestinal Pathophysiology in a Drosophila Model of Cystic Fibrosis
Lane, E.; Petsakou, A.; Liu, Y.; Chen, W.; Qadiri, M.; Hu, Y.; Perrimon, N.
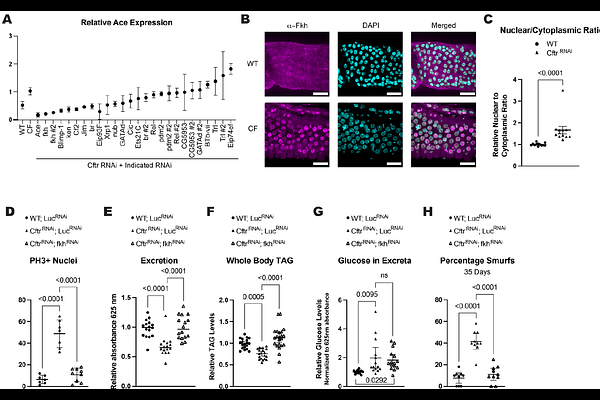
AbstractCystic Fibrosis (CF) is a monogenic genetic disease caused by mutations in the Cystic Fibrosis Transmembrane conductance Regulator (CFTR) chloride/bicarbonate channel, which is expressed in certain epithelia cells. Current therapies focus on restoring CFTR function but many gut-related pathologies persist, highlighting the need for complementary treatments to improve the quality of life of patients living with CF. In this study, we use Drosophila melanogaster as a model to investigate the gut-specific effects of Cftr loss. We demonstrate that enterocyte-specific knockdown of Cftr in flies recapitulates several CF pathologies, including reduced intestinal motility, nutrient malabsorption, and decreased energy stores. Using single-nuclei RNA sequencing (snRNA-seq), we identify significant transcriptional changes in the CF model gut, including the upregulation of acetylcholine esterase (Ace, human AChE), which leads to reduced cholinergic signaling. Cholinergic signaling has been shown to affect CFTR function but this is the first time CFTR loss of function has been shown to alter cholinergic signaling. Functional assays confirm that cholinergic sensitivity is diminished in CF guts and restoring cholinergic signaling via Ace knockdown rescues multiple CF-associated phenotypes. Furthermore, we identify the transcription factor Forkhead (Fkh), the Drosophila homolog of human FOXA1/FOXA2, which is known to be a positive regulator of Cftr in the intestine, as a positive regulator of Ace expression in CF guts. This study establishes the Drosophila gut as a powerful model to investigate CF pathogenesis, genetic modifiers, and identifies Ace and Fkh as genetic modifiers. This work also suggests that enhancing cholinergic signaling may represent a viable therapeutic strategy for gastrointestinal manifestations of CF.