BST-2 inhibits SARS-CoV-2 egress at intracellular membranes and is neutralized by ORF7a
BST-2 inhibits SARS-CoV-2 egress at intracellular membranes and is neutralized by ORF7a
Smith, A.; Dong, X.
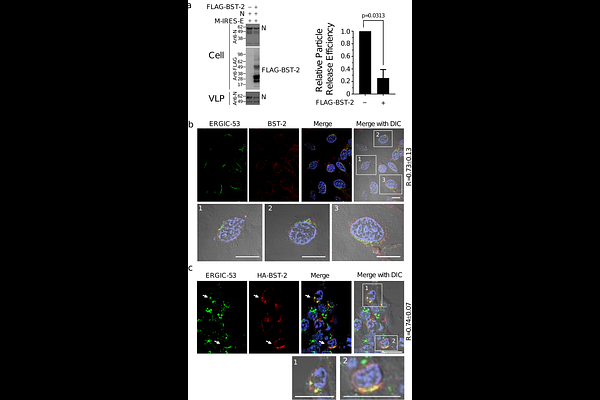
AbstractBone marrow stromal antigen 2 (BST-2, or tetherin) is an interferon-inducible host restriction factor that inhibits the release of enveloped viruses by tethering nascent virions to cellular membranes. While its antiviral function is well established in retroviral systems, its role in SARS-CoV-2 egress remains unclear. Here, we used a virus-like particle (VLP) system composed of SARS-CoV-2 structural proteins M, E, and N to investigate the impact of BST-2 on viral particle release. BST-2 significantly inhibited VLP release in HEK293T and Calu-3 lung epithelial cells. Confocal microscopy revealed that BST-2 colocalizes with viral structural proteins at the endoplasmic reticulum-Golgi intermediate compartment (ERGIC), the main site of coronavirus assembly. We next evaluated the roles of the SARS-CoV-2 accessory proteins ORF3a and ORF7a in overcoming this restriction. ORF3a localized to endolysosomal compartments and promoted VLP release through a BST-2-independent mechanism, without altering BST-2 expression or localization. In contrast, ORF7a colocalized with both BST-2 and ERGIC markers and restored VLP release by promoting BST-2 degradation. Notably, ORF7a also relieved BST-2-mediated restriction of HIV-1 VLP release, suggesting a conserved antagonistic function. These findings identify BST-2 as an intracellular inhibitor of SARS-CoV-2 particle release and establish ORF7a as viral accessory antagonist that neutralizes this host defense.