An approach to differentiating biological from chemical sulfide oxidation on corroded concrete sewer surfaces
An approach to differentiating biological from chemical sulfide oxidation on corroded concrete sewer surfaces
Huang, X.; Yin, Y.; Zuo, Z.; Shi, T.; Kuen, T.; Zheng, M.
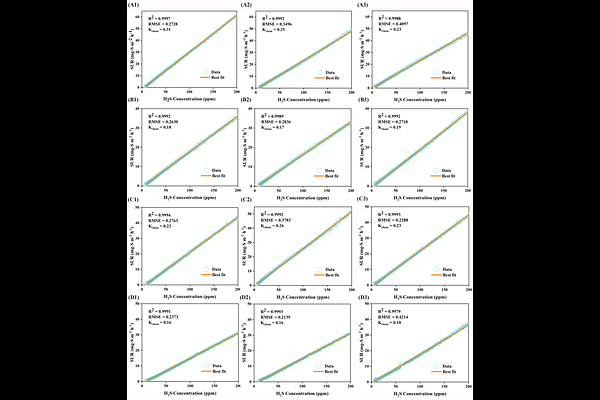
AbstractHydrogen sulfide (H2S)-induced sewer corrosion largely impacts the longevity of infrastructure worldwide. The corrosion incorporates two pathways, i.e., chemical and biological oxidation of H2S to sulfuric acid; however, their rates are difficult to differentiate on corroded sewer pipelines, limiting understanding and application of effective strategies for corrosion control. Here, this study develops and reports a kinetic approach to determining rates of chemical and biological sulfide oxidation. By measuring the total sulfide uptake rate of sewer corrosion concretes, extensive experimental data was obtained for calibration and validation of the proposed kinetic model. The modelling results fit with measured data indicating that both chemical and biological sulfide oxidation can be well-described by the developed model. Model predictions revealed that along with sewer corrosion (i.e., pH decrease), chemical sulfide oxidation rate was significantly accelerated, along with an increase in biological rate. This result suggests that the chemical sulfide oxidation process should not be overlooked for seriously corroded concretes, and synergistic strategies to suppress both biological and chemical rates are required. Specifically, the chemical process can predominate sulfide oxidation with a gas-H2S concentration higher than 300 ppm. The work provides a useful tool for future corrosion studies and also valuable insights for sewer corrosion management practices.