Nitrogen deletion in the biosynthesis of HDAC-targeting anticancer drugs
Nitrogen deletion in the biosynthesis of HDAC-targeting anticancer drugs
Jian, X.; Zhao, J.; Marschall, E. M.; Roberts, D. M.; Cryle, M. J.; Alkhalaf, L. M.; Challis, G.
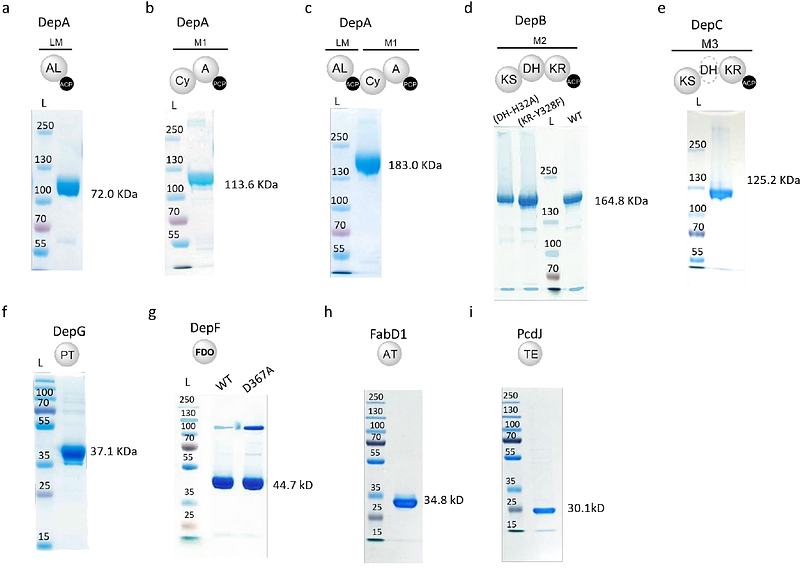
AbstractNatural product biosynthetic pathways employ diverse enzymatic strategies to perform site-specific modifications on molecular scaffolds, tailoring their bioactivity. Here, we uncovered an unprecedented nitrogen deletion mechanism in the biosynthesis of the conserved pharmacophore of a depsipeptide histone deacetylase (HDAC) inhibitor family, exemplified by the clinical used drug romidepsin, revealing novel catalytic functions within polyketide synthase and nonribosomal peptide synthetase assembly lines. Our findings demonstrate that a pair of multifunctional ketoreductase and dehydratase domains, coupled with a trans-acting phosphotransferase and a flavin-dependent oxidoreductase, coordinate a series of transformations to remove the nitrogen introduced by cysteine moiety in the pharmacophore of this family HDAC inhibotors. Furthermore, we identified a novel condensation domain mediated a cryptic S-acylating protection mechanism in the biosynthesis of the conserved pharmacophore. Leveraging these insights, we established a highly efficient, one-pot enzymatic synthesis of the intact pharmacophore in its protected form, laying the groundwork for its scalable biocatalytic production. Our work expanded the mechanistic understanding of natural product site-specific editing enabled by novel functions of assembly line biosynthetic machineries and offers new avenues for biocatalytic strategies in development of this family HDAC inhibitors.