Administration of barcoded AAV capsid library to the putamen of non-human primates identifies variants with efficient retrograde transport
Administration of barcoded AAV capsid library to the putamen of non-human primates identifies variants with efficient retrograde transport
Dzhashiashvili, Y.; McBride, J. L.; Fabyanic, E.; Huang, X.; Kelly, B. M.; Walton-Gibbs, G. D.; Nayal, M.; Hippen, A. A.; Yu, Z.; Raman, P.; Ramsburg, E.; Davidsson, M.; Engel, E. A.; Bjorklund, T.
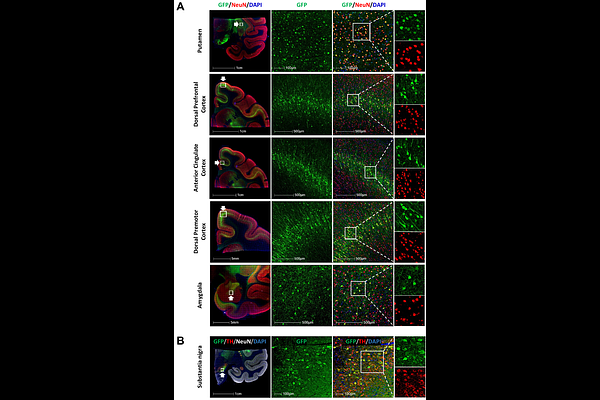
AbstractAdeno-associated viral vectors have become a leading choice for gene therapy in the central nervous system due to their safety profile, efficient neuronal transduction, and capacity for sustained transgene expression. We previously reported that AAV2-derived capsids developed using the BRAVE (Barcoded Rational AAV Vector Evolution) approach have enhanced retrograde transport properties in the rodent brain, compared to parental AAV2. Retrograde transport enables broader coverage of connected brain regions after a single focal intraparenchymal brain injection and is therefore a powerful tool for delivery of vectors to distant sites with potentially higher specificity, transduction efficacy and safety. Because transport properties can vary among species, we further characterized a barcoded library of 25 BRAVE-derived AAV2 capsid variants, along with the parental AAV2 serotype and benchmark AAV capsids, in brains of adult cynomolgus monkeys after intraputaminal dosing. Based on RNA and DNA amplicon sequencing, single-nucleus RNA sequencing, and histological assessment, we report here capsid variants with enhanced retrograde transport and expression compared to the parental AAV2 capsid. These properties make them potentially useful for disease indications in which broader brain coverage is desirable beyond the injection site.