CARM1-mediated methylation controls interactions of ALIX with key partners important for cytokinesis
CARM1-mediated methylation controls interactions of ALIX with key partners important for cytokinesis
Huard, S.; Suresh, S.; Vayr, J.; Ruggiero, S.; Dakroub, R.; Masson, V.; Petrovic, A.; Dibsy, R.; Thomsen, J.; Hajj Younes, Y.; Vissotsky, C.; Amara, L.; Belmudes, L.; Chatellard, C.; Sadoul, R.; El Marjou, A.; Coute, Y.; Loew, D.; Guerois, R.; Reynoird, N.; Echard, A.; Dubois, T.
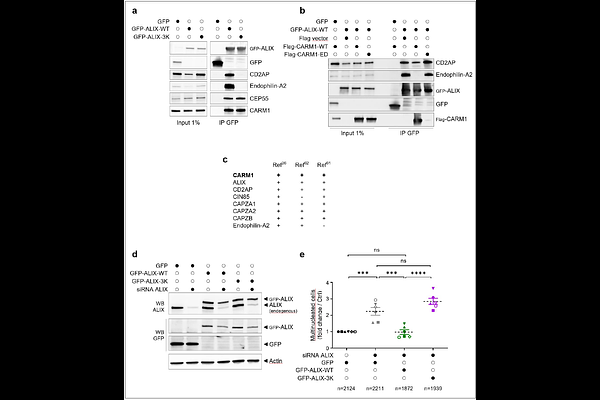
AbstractCARM1 is an arginine methyltransferase with a well-established role in regulating gene expression, but its cytoplasmic functions remain largely uncharacterized. Here, we identified ALIX, a protein acting with the ESCRT-III machinery in numerous membrane remodeling events, as a main cytoplasmic partner and a relevant substrate of CARM1. We demonstrate that CARM1 methylates arginine residues within the proline-rich motif of ALIX. At the molecular level, ALIX methylation impairs SH3-dependent association with CD2AP, CIN85, and endophilin-A2 that are required for cytokinesis. Using a mutant of ALIX that is unable to bind these proteins, we further show that these interactions are essential for ALIX functions during cytokinesis. Altogether, this work highlights the significance of arginine methylation as a regulatory post-translational modification important for the final step of cell division, by modulating interactions between proline-rich domains and their SH3-containing partners.