Mini-bacterioferritins: Structural insight into a new type of ferritin-like protein from an anaerobic methane-oxidising archaeon
Mini-bacterioferritins: Structural insight into a new type of ferritin-like protein from an anaerobic methane-oxidising archaeon
Wissink, M.; Engilberge, S.; Leao, P.; Jansen, R. S.; Jetten, M. S. M.; Royant, A.; Welte, C. U.; Wagner, T.
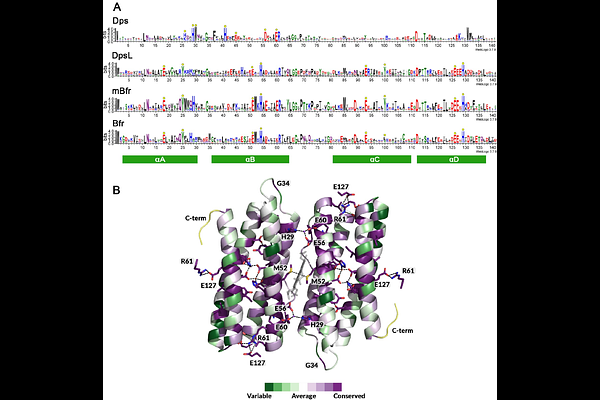
AbstractFerritins are ubiquitous among life forms, as they are essential for iron homeostasis. Here, we unveiled a novel member of the ferritin family, baptised mini-bacterioferritin. The characterised mini-bacterioferritin was isolated from a microbial enrichment dominated by the methanotrophic archaeon \"Candidatus Methanoperedens\" BLZ2. Its atomic resolution crystal structure reveals a 12-mer assembly with a diiron ferroxidase centre located within a four-helix bundle. Redox cycling experiments on protein crystals reveal a shift in iron position at the active site, which follows the established ferritin catalytic cycle. The 12-mer sphere-like structure harboured six Fe-coproporphyrin III ligands, positioned at the interdimeric interface, a characteristic previously only found in 24-mer bacterioferritins. Phylogenetics, together with structure predictions of closely related proteins, revealed that mini-bacterioferritins form a new clade within the ferritin family that might conserve ancestral traits. Future research will need to investigate the physiological roles of these enzymes, which were unsuspectingly widely distributed among prokaryotes.