Tbr2-Dependent Parallel Pathways Regulate the Development of Distinct ipRGC Subtypes
Tbr2-Dependent Parallel Pathways Regulate the Development of Distinct ipRGC Subtypes
Kiyama, T.; Chen, C.-K.; Altay, H. Y.; Chen, Y.-J.; Sigala, L.; Su, D.; Eliason, S. L.; Amendt, B.; Mao, C.-A.
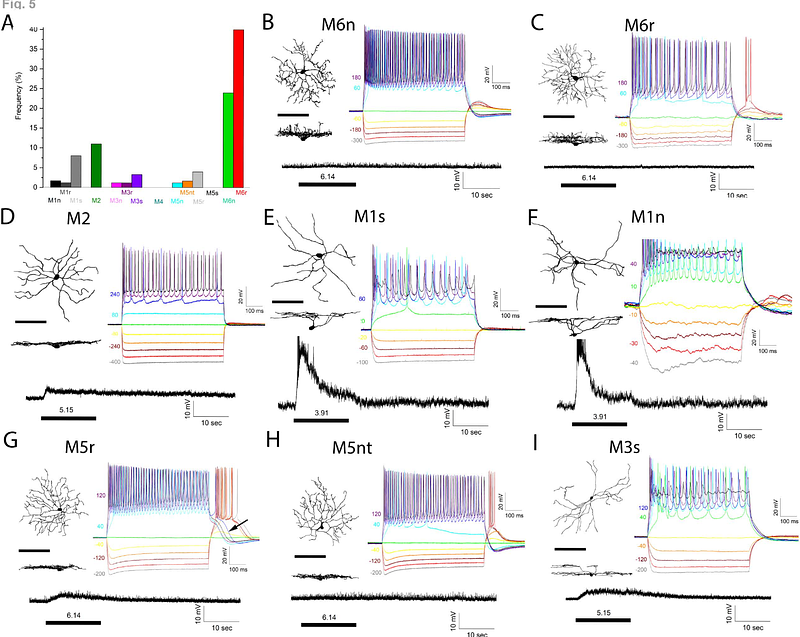
AbstractThe intrinsically photosensitive retinal ganglion cells (ipRGC) are the conduit between the retinas and brain regions responsible for non-image-forming and image-forming vision. In mice, six ipRGC subtypes have been discovered based on morphological characteristics, functions, and molecular profiles. All ipRGCs arise from Tbr2-expressing RGCs during developmental stages and subsequently diverge and differentiate into the six mature, distinct subtypes in adult retinas. However, the cellular and molecular mechanisms controlling the formation and maturation of the six ipRGC subtypes remain elusive. Here, we demonstrate that two Tbr2-dependent transcription factors, Iroquois-related homeobox 1 (Irx1) and T-box containing factor 20 (Tbx20), are key downstream transcription factors guiding lineage segregations of Tbr2-expressing RGC into distinct adult ipRGC subtypes. Both factors also control Opn4 expression. Irx1 is expressed in the M3, M4, and M5 subtypes, while Tbx20 is predominantly expressed in M1, M2, M6, and subgroups of M3 and M5. When Irx1 is ablated during retinal development, Opn4 expression is significantly reduced in the M3, M4, and M5 ipRGC groups; however, the formation of Irx1-expressing ipRGCs is not affected. In contrast, when Tbx20 is deleted, a significant number of Tbx20-expressing cells fail to develop while Opn4 expression is down-regulated. These findings reveal two parallel transcription cascades downstream of Tbr2 for controlling ipRGC subtype formation, fate divergence, and maintenance in the adult retina.