Analysis of apo and citraconate-bound hACOD1 (hIRG1) by X-ray crystallography and NMR spectroscopy: structural insights for developing chemotherapeutic agents
Analysis of apo and citraconate-bound hACOD1 (hIRG1) by X-ray crystallography and NMR spectroscopy: structural insights for developing chemotherapeutic agents
Monteiro, D. C. F.; Runge, B.; Fucci, I. J.
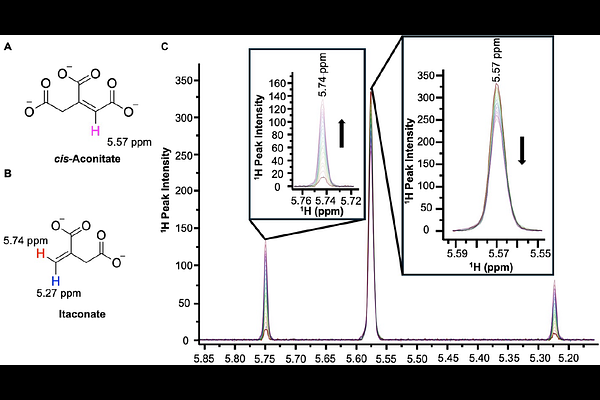
AbstractIn this work, we present the high-resolution structure of human aconitate decarboxylase 1 (hACOD1) in its true apo form (active site empty) as well in complex with the inhibitor citraconate. These two new structures show the architecture of the active site and the structure-activity relationships of citraconate inhibition. Careful analysis of the structures indicates probable dynamics required for substrate/inhibitor binding and catalysis. These observations were further explored using molecular dynamic simulations, which show a clear open-close mechanism of hACOD1 between the A1 and A2 loops, the lid- and helical-domain respectively. As part of the biochemical characterization of the protein, we also developed an alternative kinetic assay which measures the rate of catalysis of hACOD1 by direct observation of the conversion of cis-aconitate to itaconate by NMR spectroscopy. The work herein offers a foundation for structure- and dynamic-driven design of novel hACOD1 inhibitors as novel chemotherapeutics.