HPDL is critical in human cortical development via regulation of mitochondrial functional properties
HPDL is critical in human cortical development via regulation of mitochondrial functional properties
Baggiani, M.; Desbats, M. A.; Naef, V.; Giacich, M.; Galatolo, D.; Mero, S.; Zampieri, S.; Cappello, V.; Valentino, A.; Salviati, L.; Santorelli, F. M.; Damiani, D.
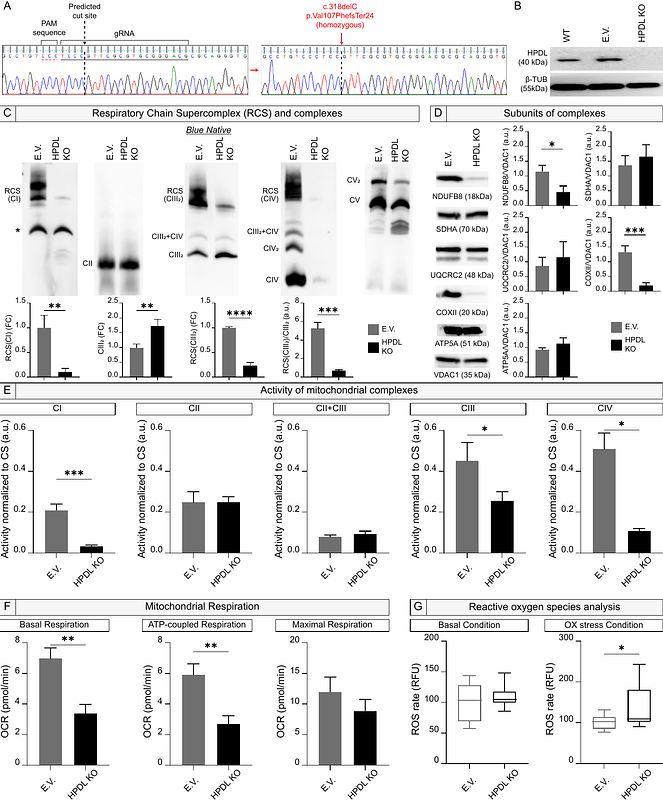
AbstractHuman brain development is highly regulated by several spatiotemporal processes, which disruption can result in severe neurological disorders. Emerging evidence highlights the pivotal role of mitochondrial function as one of these fundamental pathways involved in neurodevelopment. Our study investigates the role of 4-hydroxyphenylpyruvate dioxygenase-like (HPDL) protein in cortical neurogenesis and mitochondrial activity, since mutations in the HPDL gene are associated with SPG83, a childhood-onset form of hereditary spastic paraplegia characterized by corticospinal tract degeneration and cortical abnormalities. Starting from mutant neuroblastoma cells, we demonstrated that HPDL is essential to mitochondrial respiratory chain supercomplex assembly and cellular redox balance. Moreover, transcriptomic analyses revealed dysregulated pathways related to neurogenesis, implicating HPDL role in early cortical development. To further elucidate the role of HPDL, we generated cortical neurons and organoids from SPG83 patient-derived induced pluripotent stem cells. Mutant cells exhibited premature neurogenesis at early differentiation stages, likely leading to depletion of cortical progenitors, as evidenced by decreased proliferation, slight increase of apoptosis, and unbalanced cortical type composition at later stages. Furthermore, cortical organoids derived from SPG83 patients showed impaired growth, reminding microcephaly observed in severe cases. In addition, mitochondrial morpho-functional characterization in mutant neurons confirmed disruption of OxPhos chain functionality and increased ROS generation rate. Treatment of cortical cells with two antioxidant compounds, could partially revert premature neurogenesis. In conclusion, our findings reveal a critical role for HPDL in coordinating cortical progenitor proliferation, neurogenesis, and mitochondrial function. These insights shed light on a mechanistical understanding of SPG83 pathology and underscore the therapeutic potential of targeting oxidative stress in this and related neurological disorders.