Historical Contingency Shapes Toxin Resistance in a Specialist Avian Predator
Historical Contingency Shapes Toxin Resistance in a Specialist Avian Predator
Mohammadi, S.; Pradhan, S.; Hoffmann, F. G.; Herrera-Alvarez, S.; Deng, Y.; Eacock, A.; Dobler, S.; Storz, J. F.; Rowland, H. M.
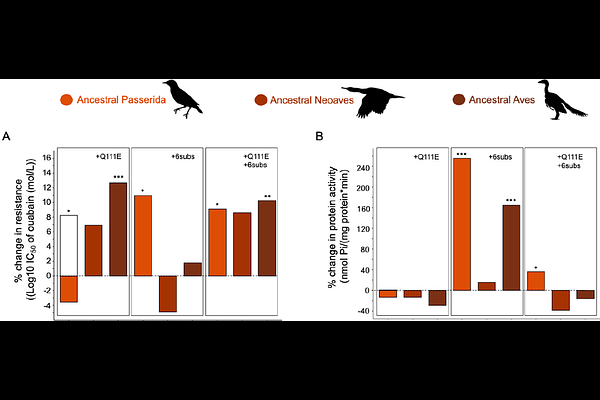
AbstractAdaptations to toxic diets can cascade through ecosystems, altering physiology, species interactions, and trophic dynamics. To uncover the molecular basis of toxin resistance in the black-headed grosbeak (Pheuticus melanocephalus), a specialist predator of cardiotonic steroid-defended monarch butterflies (Danaus plexippus), we investigated the evolution of target-site insensitivity in the toxin\'s molecular target--the Na,K-ATPase. Functional assays of engineered Na,K-ATPases revealed that resistance in grosbeaks arises from a non-additive interaction between a substitution at position 111 (Q111E) and up to six nearby amino acid changes in the first extracellular loop of the protein. Using resurrected ancestral proteins, we show that the earliest of these six substitutions to evolve (V113L) altered the functional effects of Q222E, such that Q111E alone became maladaptive. Only after the accumulation of additional permissive substitutions could Q111E confer resistance, highlighting how intramolecular epistatic interactions and historical contingency constrained the evolutionary path to adaptation. Our phylogenetic analysis of Na,K-ATPase sequences from 360 birds further revealed that several of the specific grosbeak substitutions--particularly at sites 112, 114, and 116--show strong signatures of co-evolution with changes at site 111 across the avian tree, supporting the hypothesis that resistance evolves through repeated, interacting changes. Together, these results reveal the molecular mechanisms of convergent evolution of toxin resistance and demonstrate how genetic background can shape evolutionary outcomes across trophic levels.