Mono-Ubiquitylation-Dependent Rap2 Activation Regulates Lamellipodia Dynamics During Cell Migration
Mono-Ubiquitylation-Dependent Rap2 Activation Regulates Lamellipodia Dynamics During Cell Migration
Neumann, A. N. J.; Sampath, r.; Mayerhofer, E.; Mikalayeva, V.; Skeberdis, V. A.; Sarapiniene, I.; Prekeris, R.
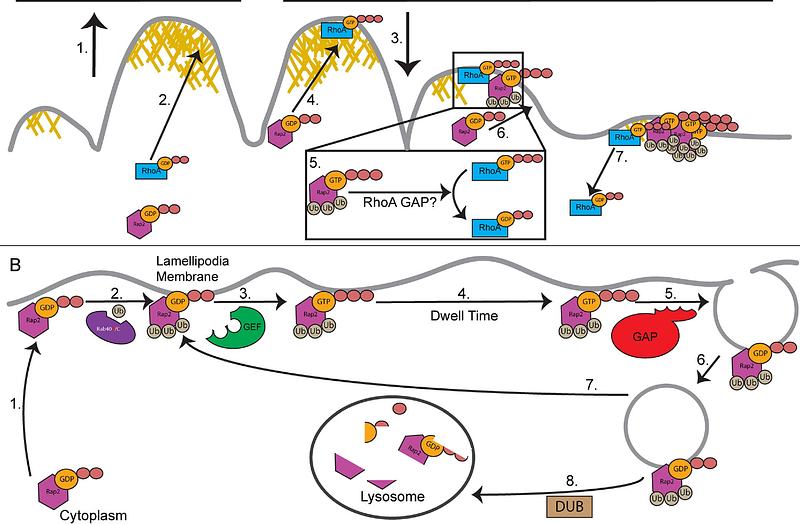
AbstractCell migration is a complex process hallmarked by front-to-back cell polarity that is established by the highly dynamic actin cytoskeleton. Branched actin polymerization creates a lamellipodia at the leading edge of the cell, while the contractile acto-myosin cytoskeleton is present at the lagging edge. Rap2, a Ras GTPase family member, has previously been reported to localize to the lamellipodia as a result of Rab40/CRL5 E3 ubiquitin ligase induced tri-mono-ubiquitylation. However, how Rap2 functions and how mono-ubiquitylation targets Rap2 to the lamellipodia remained unclear. Here, we demonstrate that Rap2 is recruited to retracting lamellipodia ruffles where it inhibits RhoA and regulates lamellipodia dynamics and facilitates cell migration. Furthermore, using a variety of genetic and pharmacological techniques, we show that tri-mono-ubiquitylation is required for GEF-dependent Rap2 activation, a necessary step for Rap2 targeting to lamellipodia membrane. As such, we demonstrate how this unique mono-ubiquitylation of Rap2 regulates lamellipodia actin dynamics during cell migration.