Myo1e/f at the podosome base regulate podosome dynamics and promote macrophage migration
Myo1e/f at the podosome base regulate podosome dynamics and promote macrophage migration
Cervero, P.; Barger, S. R.; Verdys, P.; Herzog, R.; Paul, T.; Palmieri, M.; Poincloux, R.; Linder, S.; Krendel, M.
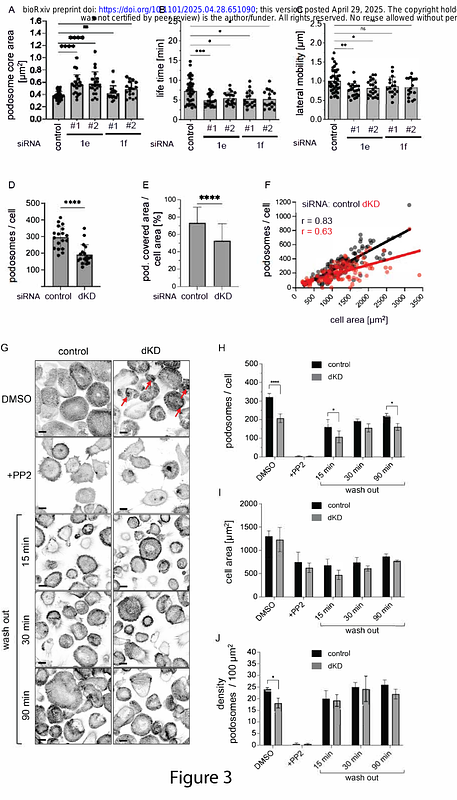
AbstractCells of the monocyte lineage form specialized membrane-associated, actin-rich structures, called podosomes. Podosomes play important roles in cell adhesion and migration as well as the proteolytic degradation of the extracellular matrix. While podosomes are always closely associated with the plasma membrane, the structural components linking the podosome core, composed of branched actin, to the membrane are not fully understood. In this study we show that class I myosins, Myo1e and Myo1f, localize to a specific region of podosomes, underneath the podosome core and near the ventral plasma membrane, and that this localization is mainly mediated by the Myo1e/f TH2 domains. Respective knockdowns or knockouts of Myo1e/f lead to increased podosome size, altered turnover and lateral mobility, which is likely due to Myo1e/f regulating the attachment of core actin filaments to the plasma membrane. In addition, Myo1e/f double knockout macrophages were characterized by a reduction in 3D and 2D migration, even though these cells exhibited increased ability to degrade the extracellular matrix. Along with the other membrane-associated podosome components, such as the transmembrane proteins MT-MMP and CD44, and the GPI-anchored DNase X, Myo1e and Myo1f mark the membrane-proximal region of podosomes. We propose to label this region as the podosome \"base\", an additional substructure joining the current trifecta of the podosome cap, core, and ring.