Transglutaminase 2 is an RNA-binding protein: Experimental verification and characterisation of a novel transglutaminase feature
Transglutaminase 2 is an RNA-binding protein: Experimental verification and characterisation of a novel transglutaminase feature
Csaholczi, B.; Csuth, A. R.; Korponay-Szabo, I. R.; Fesüs, L.; Kiraly, R.
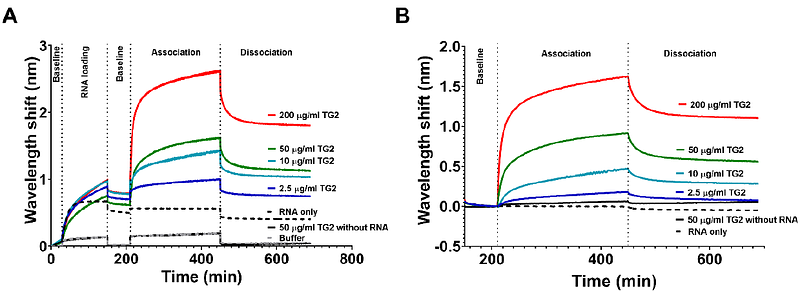
AbstractTransglutaminase 2 (TG2) is a uniquely versatile protein with diverse catalytic activities, such as transglutaminase, protein disulfide isomerase, GTPase, protein kinase, and participates in several biological processes. According to information available in the RBP2GO database, TG2 can be an RNA-binding protein (RBP). RBPs participate in posttranscriptional gene expression regulation, influencing RNAs\' function, while RNA molecules can also modulate RBPs\' biological activity. Our goal was to confirm this novel character of TG2 in human umbilical cord vein endothelial cells (HUVEC), which physiologically express TG2. First, UV cross-linked RNA-protein complexes were isolated from immortalised HUVEC using orthogonal organic phase separation. Compared with the RBP2GO database, mass spectrometry identified 392 potential RBPs, including TG2 and 20 novel, endothelium-related RBPs. Total RNA from HUVEC pulled down recombinant human TG2. Complex formation between TG2 and a 43-mer RNA molecule with a secondary structure as well as a homo-oligomeric single-stranded poly(dG), but not poly(dA), could be observed in magnetic RNA-protein pull-down experiments. Experiments with TG2 inhibitors NC9 and GTP{gamma}S, which stabilise its open and closed conformation, respectively, revealed that the open conformation of the enzyme favoured RNA-binding. Biolayer interferometry revealed a high binding affinity between TG2 and RNA with a KD value of 88 nM. We propose that superficial residues on the catalytic core and C-terminal {beta}-barrel domains, being in a hidden position in the closed TG2, are involved in RNA binding. Our study demonstrates TG2\'s previously uncharacterised RNA-binding ability, opening new avenues for understanding its multi-functionality.
