The V-ATPase/ATG16L1 axis drives membrane remodeling during epithelial morphogenesis
The V-ATPase/ATG16L1 axis drives membrane remodeling during epithelial morphogenesis
Baonza, G.; Alfonso, T.; Herranz, G.; Quintana-Quintana, C.; Gordillo-Vazquez, C.; El Mazjoub, Y.; Escudero, L. M.; Miguez, D. G.; Marti, E.; Martinez-Martin, N.; Martin-Belmonte, F.
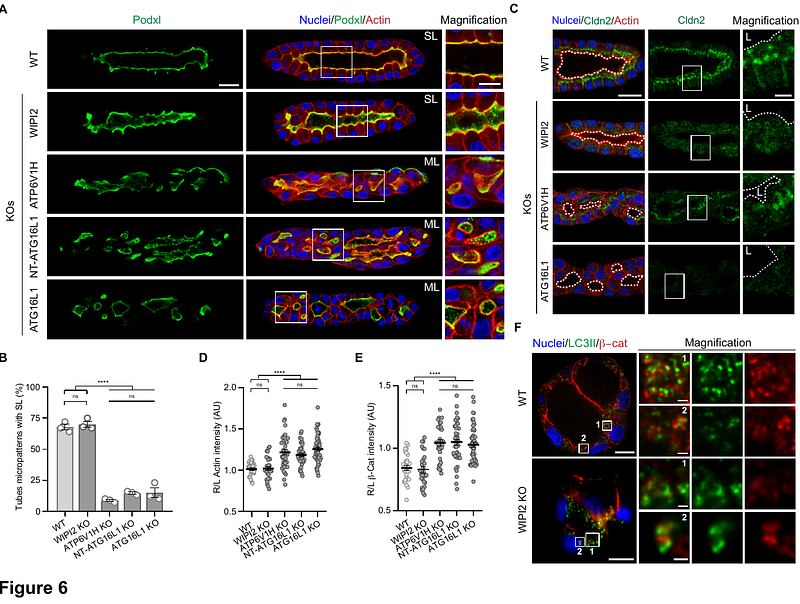
AbstractEpithelial tubulogenesis shapes internal organs by transforming flat epithelial sheets or unpolarized cords into hollow tubes with central lumens. A key example is the formation of the posterior neural tube during the secondary neurulation, which requires precise morphogenetic events for de novo lumen formation. Although several studies have highlighted the role of autophagy in specific morphogenetic events, its involvement in epithelial organ development remains unclear. Autophagy operates via canonical and noncanonical pathways. Canonical autophagy is catabolic, requiring double-membrane autophagosomes and the full ATG protein set. Noncanonical autophagy, including the V-ATPase/ATG16L1-dependent Conjugation of ATG8 in Single Membranes (CASM), has both degradative and non-degradative roles and regulates different membrane trafficking processes. Using human neural tube organoids, spheroids, and tube micropatterns deficient in CASM or canonical autophagy, we show that CASM plays a pivotal role in epithelial tube morphogenesis. Specifically, the V-ATPase/ATG16L1 axis is essential for de novo lumen formation by regulating membrane junction remodeling and Rab11-dependent recycling pathways. These findings reveal distinct contributions of autophagy pathways in epithelial development, with potential implications for diseases linked to autophagy dysfunction.