Salt-inducible kinase inhibition promotes weight loss and improves the diastolic function of obesity-related heart failure with preserved ejection fraction in mice
Salt-inducible kinase inhibition promotes weight loss and improves the diastolic function of obesity-related heart failure with preserved ejection fraction in mice
Shi, F.; Agrawal, V.; Munkhjargal, U.; Kobeck, E.; Patel, H. U.; Zhang, W.; Collins, S.
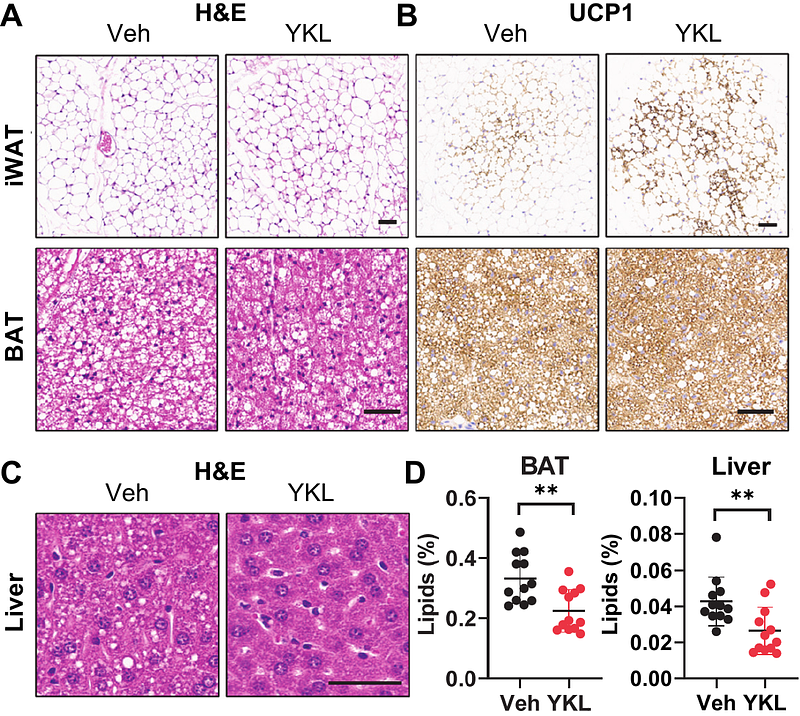
AbstractBackground: Obesity negatively impacts cardiac function and is closely associated with heart failure with preserved ejection fraction (HFpEF). Salt-inducible kinases (SIKs) are critical regulators of energy metabolism and cardiovascular function. Small molecule SIK inhibitors have been developed but their effect in treating obesity-related HFpEF remains unexplored. We recently discovered that pharmacological SIK inhibition promotes the adipose tissue thermogenesis and mitochondrial biogenesis gene program. We reason that targeting SIKs to treat obesity-related complications would be beneficial for HFpEF. Methods: We employed a preclinical HFpEF mouse model induced by two-hit stress of high-fat diet (HFD) and nitric oxide synthase (NOS) inhibition using L-NAME in drinking water. Eight-week-old C57BL/6J mice received regular low-fat chow diet or HFD/L-NAME for 5 weeks and treated with vehicle or a pan-SIK inhibitor YKL-05-099 (YKL) via daily intraperitoneal injection in the last 4 weeks. Body weight and parameters of adiposity, energy balance and glucose tolerance were assessed. Cardiovascular function was characterized by echocardiography and in vivo pressure-volume loop hemodynamic analysis. Myocardial transcriptomic data were analyzed to determine if changes of SIK gene expression are associated with human HFpEF. Results: YKL treatment limits body weight gain mainly by reducing the fat mass in obese HFpEF mice. YKL-treated mice show better glucose tolerance, enhanced adipose tissue browning and decreased lipid deposition. YKL-treatment mice demonstrate preserved left ventricular (LV) ejection fraction, reduced LV filling pressure and improved diastolic function. Myocardial expression of SIK1, SIK2, and SIK3 mRNA is down-regulated in patients with HFpEF. However, higher SIK mRNA expression is associated with a subgroup of HFpEF patients that has a greater risk for HF hospitalization and/or death. Conclusions: Taken together, our study reveals a pathological role for SIKs in obesity-related HFpEF and suggests that pharmacological SIK inhibition would be a disease-modifying strategy for obese HFpEF, for which evidence-based therapy has been limited.