Human CXCL17 Activates and Binds to Fish GPR25 Orthologs
Human CXCL17 Activates and Binds to Fish GPR25 Orthologs
Hu, W.-F.; Wang, J.-J.; Yu, J.; Yao, J.-J.; Liu, Y.-L.; Xu, Z.-G.; Guo, Z.-Y.
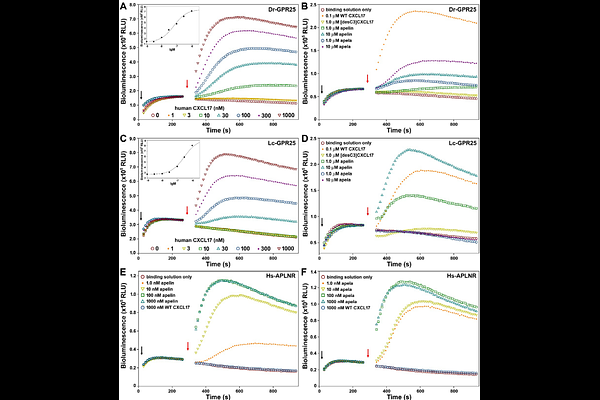
AbstractC-X-C motif chemokine ligand 17 (CXCL17) functions as a chemoattractant, though its receptor has been controversial. Recent independent studies, including our own, identified CXCL17 as an agonist for the orphan G protein-coupled receptor 25 (GPR25). While GPR25 orthologs are found across fishes to mammals, CXCL17 orthologs appear to be mammalian-specific, leaving the endogenous ligand for non-mammalian GPR25 orthologs unknown. This study unexpectedly found that human CXCL17 exhibits high activity towards GPR25 orthologs from the zebrafish (Danio rerio) and coelacanth (Latimeria chalumnae). Recombinant human CXCL17 efficiently activated both fish GPR25 orthologs in a NanoLuc Binary Technology (NanoBiT)-based {beta}-arrestin recruitment assay, and induced chemotactic movement in transfected human embryonic kidney (HEK) 293T cells expressing fish GPR25. A human CXCL17 mutant lacking three C-terminal residues showed no such effect. A NanoBiT-based binding assay revealed that a SmBiT-tagged human CXCL17 C-terminal fragment specifically bound to secretory large NanoLuc fragment (sLgBiT)-fused fish GPR25 orthologs. Fish GPR25 orthologs had significantly higher cell surface expression in transfected HEK293T cells compared to human GPR25, improving {beta}-arrestin recruitment assay data quality. Despite approximately 400 million years of divergence between humans and fishes, the high activity of human CXCL17 on fish GPR25 orthologs suggests that the CXCL17-GPR25 pair may be conserved across all vertebrates, even though non-mammalian CXCL17 orthologs remain unidentified.