Interventionally targeting somatic CAG expansions can be a rapid disease-modifying therapeutic avenue: Preclinical evidence.
Interventionally targeting somatic CAG expansions can be a rapid disease-modifying therapeutic avenue: Preclinical evidence.
Gall-Duncan, T.; Ko, S. Y.; Quick, I. K.; Khan, M.; Feng, K.; Kelley, C. P.; Coleman, A.; Touze, A.; Tang, S.; Mehkary, M.; Yokoi, K.; Herrington, C. R.; You, J.; Lambie, S. C.; Prasolava, T. K.; Panigrahi, G. B.; Park, J.; Nakatani, K.; Byrne, L. M.; Wang, P.; Schneekloth, J. S.; Nakamori, M.; Frankland, P. W.; Pearson, C. E.
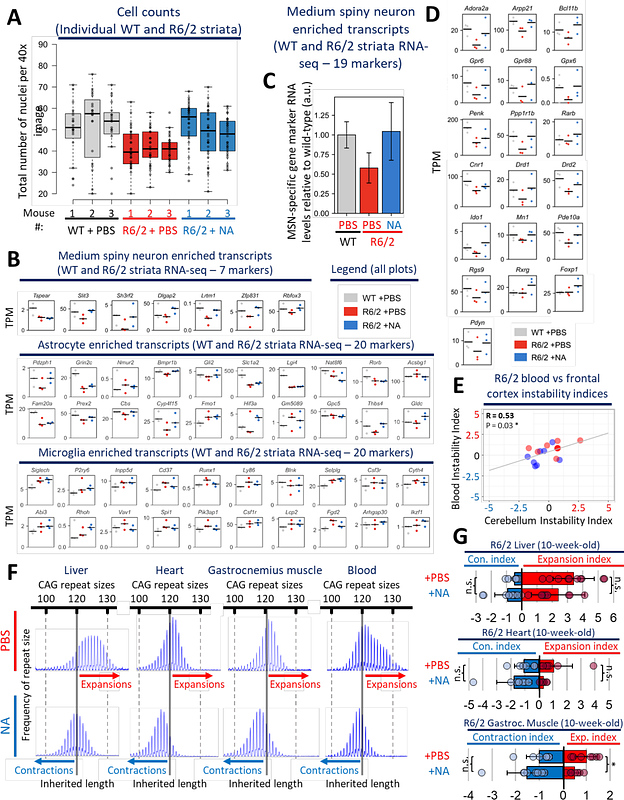
AbstractHuntington disease (HD) is caused by inherited CAG expansions, which continue expanding somatically in affected brain regions to hasten disease onset and progression. Therapeutically diminishing somatic expansions is expected to be clinically beneficial. However, it is not known if interventionally modifying somatic CAG expansions will actually modify in vivo clinically-relevant phenotypes, what the therapeutic window is, or which phenotypes will be altered. Here we show that acute (6-week) delivery of the contraction-inducing slipped-CAG DNA ligand naphthyridine-azaquinolone to young (4-week-old) (CAG)120 HD mice, induces contractions throughout brain regions, improves motor function (locomotion, balance, coordination, muscle strength), molecular disease landmarks (mHTT aggregates, nuclear envelope morphology, nucleocytoplasmic mRNA transport, transcriptomic dysregulation, neuroinflammation), and neurodegeneration. Beneficial effects of modifying somatic expansions were also evident in muscle and blood, where blood CAG instability correlated with brain instability and blood serum had diminished levels of neurofilament light (a biomarker for neurodegeneration) - offering blood as having elements of target engagement and efficacy. These data support that targeting somatic repeat expansions can be a rapid disease-modifying therapeutic avenue for HD and possibly other repeat expansion diseases. Our findings support an etiologic pathway interconnected to somatic CAG expansions that will inform the design of clinical trials expecting clinical benefit by modulating somatic expansions.