Optimal Transport Model for Gas Migration in Pneumatosis Intestinalis
Optimal Transport Model for Gas Migration in Pneumatosis Intestinalis
Tozzi, A.; Minella, R.
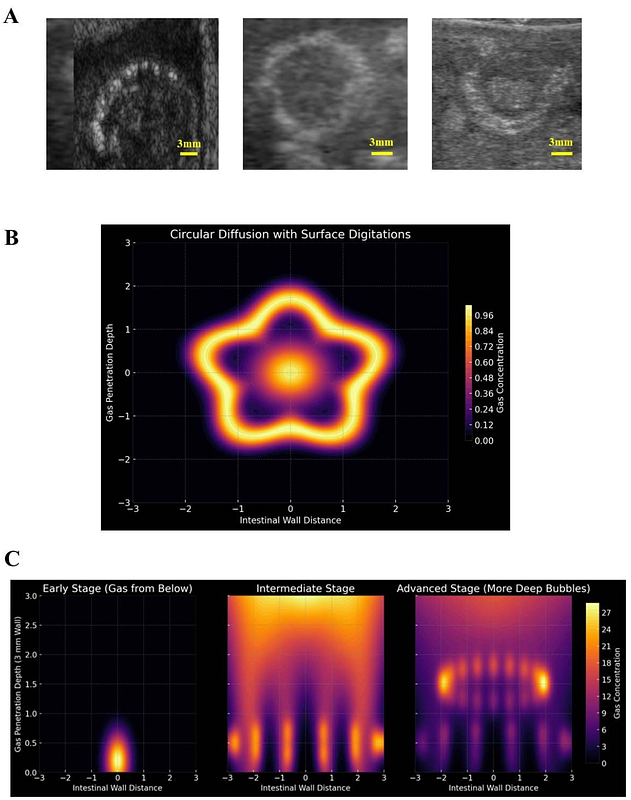
AbstractOptimal transport models may provide a robust framework for quantifying gas movement from the intestinal lumen into the gut layers in pneumatosis intestinalis (PI), a condition characterized by abnormal gas accumulation within the intestinal wall. We develop a mathematical approach that integrates circular gas diffusion from the lumen with the subsequent dispersion and entrapment of microbubbles in deeper layers. Our diffusion-advection approach establishes a radial concentration gradient that decreases with distance from the lumen. Numerical simulations illustrate the spatial distribution of gas and microbubbles within a representative three-millimeter-thick gut wall, demonstrating a transition from uniform diffusion to localized gas retention. Gas migration initially follows diffusion-driven transport but increasingly becomes influenced by local trapping mechanisms, leading to spatially heterogeneous gas retention. Microbubble formation is modeled as a function of gas entrapment in deeper tissue layers, integrating mechanical effects related to gas concentration and pressure dynamics. Additionally, our model accounts for critical biological and structural factors such as tissue permeability, mucosal integrity, bacterial fermentation, microbial overgrowth and structural heterogeneity, capturing their combined influence on gas retention. Overall, gas migration in PI follows an early-stage circular diffusion pattern, until it encounters structural resistance leading to deep microbubble formation. The density and depth of localized microbubble retention show a statistically significant correlation with gas pressure gradients and permeability variations, highlighting the interplay between transport dynamics and tissue features. Our quantitative representation provides a structured approach for refining diagnostic assessments of PI and improving the evaluation of disease severity based on underlying transport mechanisms.