Bioassembly of region-specific fibrocartilage microtissues to engineer zonally defined meniscal grafts
Bioassembly of region-specific fibrocartilage microtissues to engineer zonally defined meniscal grafts
Kronemberger, G. S.; Chattahy, K.; Spagnuolo, F. D.; Karam, A. S.; Kelly, D. J.
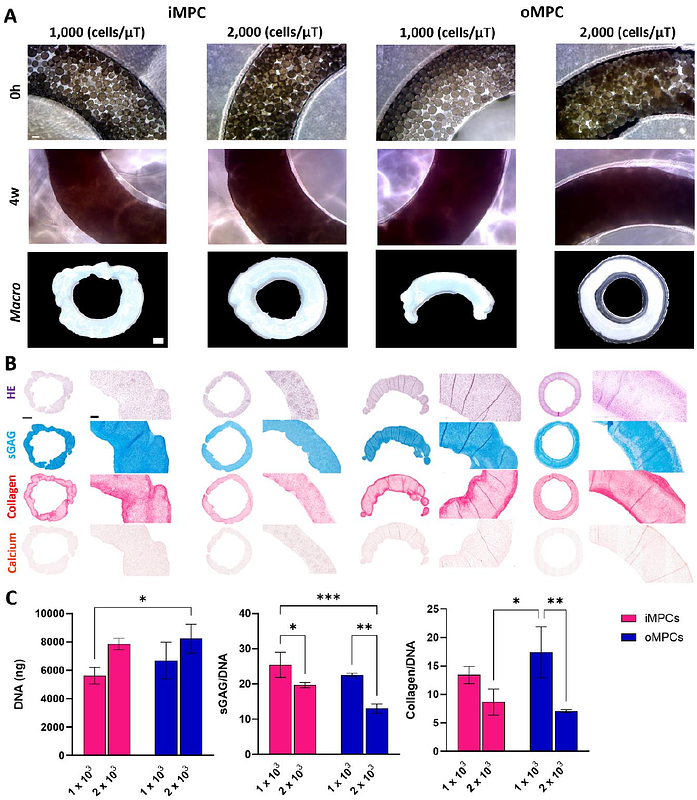
AbstractMeniscal injuries are common orthopaedic problems which can impair knee function and lead to the development of osteoarthritis. While recent advances in tissue engineering have enabled the fabrication of meniscus-like grafts, these constructs do not fully replicate the zonal structure and composition of the native meniscus. In this study, we used fibrocartilage microtissues generated from meniscus progenitor cells (MPCs) as biological building blocks to biofabricate zonally defined meniscal grafts. MPCs isolated from the inner (iMPC) and outer (oMPC) regions of caprine menisci were used to engineer region-specific meniscal microtissues in a medium-high throughput manner. Both iMPC and oMPC derived microtissues were rich in glycosaminoglycans (GAGs) and collagen, with iMPC microtissues staining more intensely for type II collagen. These microtissues were assembled into two different physically confining moulds (cylindrical and ring-shaped), where they rapidly fused and generated a fibrocartilaginous graft over six weeks of culture. Both iMPC and oMPC assembled microtissues were rich in sGAG and type I collagen, however only the iMPC assembled microtissues stained strongly for type II collagen. We then explored the impact of the catabolic enzyme chondroitinase-ABC (cABC) on the composition and structural organization of the meniscal grafts. This temporal enzymatic treatment increased collagen fiber thickness without altering tissue phenotype. Finally, iMPC and oMPC microtissues were spatially assembled to biofabricate a scaled-up and zonally defined meniscal graft. This graft was phenotypically similar to the native meniscus, with all regions rich in collagen type I and an inner core rich in collagen type II and sGAG. These findings support the use of MPC derived microtissues as biological building blocks for the engineering of zonally defined meniscal grafts.